Aleksandra ZIEMBIŃSKA, Marta SZPINDOR – Environmental Biotechnology Department, The Silesian University of Technology, Gliwice, Poland
Abstract:
Chemical disinfectants are commonly used in households, working places, educational centres etc. However, due to the fast increase of microorganisms? resistance to chemical compounds used in this type of preparations, it is important to choose a suitable and effective disinfectant that prevents bacterial infections in a fast and efficient way. In this study, an attempt was taken to estimate the effectiveness of chemical disinfection preparations: iodine tincture, solutions of salicylic alcohol, oxidized water and potassium permanganate on the autochthonous microflora of the human epidermis. Studies have revealed that iodine tincture is the most effective, while a 0.05% solution of potassium permanganate the least effective against bacteria isolated from the epidermis. Other preparations have demonstrated a surprisingly high effectiveness.
Please cite as: CHEMIK 2013, 67, 2, 127-132
Introduction
The concept of disinfection includes techniques of microorganisms control by chemical means and of their mechanical removal. Most, but not all, bacteria die during these activities. Chemical disinfectants affect vegetative forms of bacteria and of other microorganisms, while spores are most often resistant to them. Also some forms of viruses are resistant to the majority of disinfectants in use. The main goal of disinfection activities is to interrupt the route of transmission of germs between the infection source and healthy subjects. In the event of integument injury, to prevent infections the most important measure is to kill as many of microorganisms as possible on the skin, in wounds and in cavities of the human body [3].
It was not until the 19th century that antimicrobial procedures started to appear. Many antibacterial agents were created as a result of the gradual development of knowledge. A significant amount of them are disinfecting agents, which are used to remove microorganisms beyond the human body or antiseptic agents used only on the surface of body?s living tissues [4]. A suitable disinfectant should provide an effective, short-time disinfection, affect the greatest possible number of microorganism species i.e. should have a broad action spectrum, and be well tolerated by the skin, mucous membranes and wounds; it should not sensitize, and after possible absorption must not have a toxic effect. It should be characterised by long durability and undergo biological degradation. Finally, it should not have an unpleasant smell, but should undergo inactivation by blood, pus, faeces and foreign matters [8]. A wide range of disinfectants is available. Chemical disinfectants are divided into inorganic (oxidizing agents and combinations of heavy metals) and organic antiseptics (alcohols, aldehydes, acids, phenols, guanidine derivatives, ammonium compounds, sulphonamides, derivatives of nitrofuran and 8-hydroxyquinoline, quinoline and fluoroquinolones [12]).
The mechanism of action of disinfectants depends on their structure and can consist of [1, 6, 12]:
? damage of cytoplasmic membranes of microorganisms caused by a change in lipid-protein structures, a decrease in surface tension or by protein denaturation (e.g. alcohols, aldehydes, phenols) inhibition of enzymes? secretion as a result of their denaturation or
? by blocking an active medium, e.g. thiol groups (oxidizing agents, silver and mercury compounds) reacting with nucleic acids (sulphonamides, 5-nitrofuran derivatives).
Characteristic of studied disinfection compounds
Composition and mechanism of action of oxidized water
Hydrogen peroxide (hydrogenium peroxydatum) in a form of a 3% solution (oxidized water) is used to disinfect wounds. However, its antibacterial effect is very weak, mainly dehydrating and bleaching. In 30% solutions (perhydrol), it strongly irritates tissues; in such a form it is used in dentistry for cleaning the canals and in cosmetology for hair bleaching [4]. Hydrogen peroxide is an unstable product. Dry preparations containing sodium percarbonate or a combination of hydrogen peroxide with urea (Pertlenon preparation) are more stable. Upon dissolution in water, these preparations, known as ?oxidized water in tablet forms?, release hydrogen peroxide [4].
Composition and mechanism of action of salicylic alcohol
Salicylic alcohol is a 2% solution of salicylic acid in ethyl alcohol and water; it contains 2% of salicylic acid, 30% of water and 68% of ethanol as a main component. Alcohols demonstrate activity against bacteria, viruses and fungi. However, they do not affect the endospore forms. Monohydric alcohols, such as ethanol, n-propanol and isopropyl alcohol, have a stronger effect [12]. The ethyl alcohol is not too strong an antibacterial agent, but in suitable concentrations is quite effective as a bactericide against most of the common pathogenic bacteria. Some of more uncommon microorganisms survive or even proliferate in concentrations that are germicidal for other species. The effect on fungi and viruses is not certain. Ethanol does not affect spores, but inhibits their formation [4, 5].
The germicidal activity of ethyl alcohol is associated with its denaturant activity against proteins; thus, it requires water in the medium. Ethanol demonstrates the strongest disinfecting effect in a concentration of 70% in water [9, 11]. A solution of optimum concentration that contains 70% of ethyl alcohol, when applied on the skin, reduces the number of microorganisms by 90% in 2 minutes providing that at this time the skin remains damp with the alcohol. Wiping the skin with a swab once and letting it dry reduces the number of microorganisms at most by 75% [4].
Composition and mechanism of action of iodine tincture
Iodine is one of the most important disinfectants because of its fast and effective action. It has germicidal, sporicidal, fungicidal and virucidal properties and kills protozoans. Moreover, it is more comfortable to use iodine than solutions of other halogens; iodine does not have a strong reaction to the skin [8]. Iodine solutions are very often used to disinfect the skin. Iodine tincture (7?10% iodine solution in alcohol with an addition of potassium iodide) can be used to disinfect areas around wounds or non-injured skin (e.g. a surgical opening). Application of iodine tincture on the injured integuments strongly irritates the skin, causes pain and burning. If the area is wide, a huge amount of iodine can be absorbed and cause poisoning [4].
Dilute water solutions are more suitable to disinfect wounds. They can be obtained by adding water to iodine tincture to get a 0.5?1% water-alcoholic solution of iodine. Using solutions of iodine in pure alcohol, beside above mentioned disadvantages, can lead to protein coagulation on the wound surface. A denatured layer effectively protects microorganisms located in the wound from the influence of iodine [4].
Composition and mechanism of action of potassium permanganate
Potassium permanganate (kalium hypermanganicum), which is a crystal substance of a dark-purple colour, easily dissolves in water and forms solutions of the same colour. Potassium permanganate has germicidal and fungicidal properties. Effective 0.02% solutions irritate tissues, whereas in a non-irritating 0.01% concentration the germicidal effect is weak and slow. Many bacteria require the exposure time of more than 1 h. Thus, potassium permanganate is a weak agent, but in several percent solutions is a strong antibacterial agent; however, it has caustic properties and causes necrosis [4].
Characteristic of epidermis microflora
Since the moment of birth, the specific autochthonous bacterial flora starts to form on the skin surface. It consists of different microorganisms, among which staphylococci dominate: Staphylococcus albus and Staphylococcus epidermidis, as well as anaerobes occurring in the sebum that maintain system balance. Their number on the healthy skin depends on individual features of every person and an adequate hygiene. The acidity of the skin surface provides the most suitable living and reproduction conditions for skin friendly staphylococci and anaerobes in contrast to pathogenic bacteria and viruses for which only an alkaline environment is favourable. The natural bacterial flora constitutes an additional living bacteriostatic barrier, i.e. it inhibits the bacterial growth and neutralises the negative effect of bacteria.
The presence of the natural bacterial flora on the skin surface depends on many factors, such as the acidity of the skin surface, the composition of sweat and sebum or the secretion of lysozyme by skin which kills pathogenic bacteria. Factors damaging the natural bacterial flora include: changes in formation of the hydrolipidic coat as a consequence of disorders in secretion of sebum and sweat; a change of the acid reaction into the alkaline one; an antibiotic treatment; using deodorants containing antibacterial substances; skin disinfection. The physiological bacterial flora of the skin is one of its most important defence mechanisms. It should neither be removed nor destroyed by inappropriate skin care. Its loss exposes the skin to attacks of pathogenic bacteria and leads to the occurrence of different skin diseases [2].
For the whole life the man is in a close contact with numerous microorganisms that inhabit corresponding ecological niches. Human microflora is divided into two main categories [7]: the first one is formed by microorganisms that accompany the man for the whole life with some breaks, so called family microorganisms or permanent residents, and the second one by microorganisms belonging to the temporary microflora, which can come from another subject, animals or the surroundings (air, water, food etc.). The human skin has relatively low humidity and is populated mainly by Gram positive bacteria that tolerate dry environmental conditions well. Therefore, Gram negative microorganisms mostly exist in more humid parts of the body (around urinary tracts and groins) [13]. Microorganisms gather numerously on the skin around the excretory ducts of sweat and oil glands. Some of them colonise also deeper sections of oil glands. The lack of Gram negative bacteria on the major part of epidermis surface probably relates to their inability to compete for receptors located there [10].
Aim of experiment
An injury of the epidermis can result in infection by pathogenic microorganisms. However, it should be mentioned that every microorganism is potentially pathogenic, which means that even not dangerous autochthonous flora of the human epidermis can cause infections if it penetrates the wound. In such situations it is necessary to disinfect the resultant injury. However, because of a wide range of disinfectants used in disinfecting procedures, in this work, an attempt was taken to study the effectiveness of their antibacterial effect on bacteria isolated from the epidermis. Commonly used disinfectants in their working concentrations were used for analysis: 3% solution of oxidized water; 2% solution of salicylic alcohol; 3% solution of iodine tincture; 0.05% solution of potassium permanganate. The effectiveness of used disinfectants was characterised by determining a change in number of bacteria sampled before and after application of solutions of disinfectants in relation to the control sample. The analysis of bacteria number was conducted using the plate method.
Materials and methods
Bacteriological material needed for the analysis was collected from the palm epidermis surface. Bacteria were isolated by wiping the inner part of the palm surface for 1 minute with a sterile physiological saline swab. Then the swab was placed in a test tube with sterile physiological saline (9 ml) and the material was shaken for 5 minutes, which allowed us to obtain a bacterial suspension. 1 ml of the bacterial suspension was mixed at a 1:1 (v/v) ratio with solutions of disinfectants: 3% solution of oxidized water; 2% solution of salicylic alcohol; 3% solution of iodine tincture; 0.05% solution of potassium permanganate. Resultant mixtures were inoculated in four repetitions on the surface of Petri dishes with broth medium (BTL, Łódź), each dish containing 0.1 ml. The control sample was the bacterial suspension from the epidermis with no addition of disinfectants. The inoculated Petri dishes were placed in the incubator at 28?2°C (the temperature similar to that of the epidermis surface) and incubated for 24 hours. After incubation, bacteria colonies grown on plates were counted.
Results
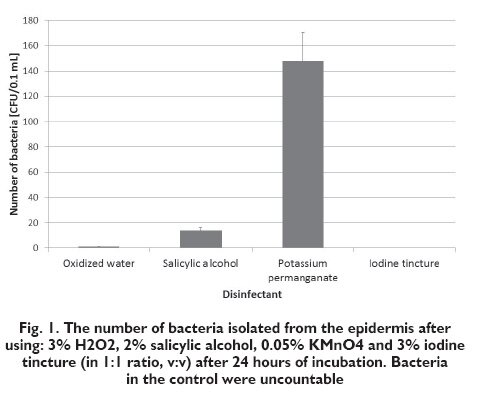
The effectiveness of antibacterial effect of selected disinfectants on bacteria isolated from the palm epidermis was analysed. Table I presents the results of the analysis of bacteria number from the palm epidermis in the control sample and after application of 3% solution of oxidized water, 2% solution of salicylic alcohol, 0.05% solution of potassium permanganate and 3% solution of iodine tincture (at a 1:1 v/v ratio). Figure 1 shows average numbers of bacteria in the control sample and after application of particular disinfectants.

Discussion of results
The base number of microorganisms in the studied sample was high (uncountable number of bacteria colonies in the control sample). After application of all studied compounds, it was significantly reduced (for the solution of iodine tincture even to zero). It is the evidence of high effectiveness of all used preparations. However, it is worth to notice that disinfectants selected for this study are characterised by different mechanisms and effectiveness of action. The conducted analysis shows that potassium permanganate, and directly after it salicylic alcohol, are characterised by the weakest germicidal effectiveness against bacteria isolated from the epidermis.
The weakest germicidal effect was demonstrated for the samples treated with potassium permanganate. It is known that in concentrations used for skin disinfection, it is a weak and slow agent; in higher concentrations it is a strong germicidal agent, but irritating for the skin. Such a low effectiveness could have resulted from the low concentration of this compound caused by its additional dilution in physiological saline. Potassium permanganate is definitely more effective in higher concentrations. The low effectiveness could have also resulted from too short reaction time before inoculating the suspension on a medium. In the samples of salicylic alcohol, a huge number of bacterial colonies grew (over 10 times more than after oxidized water application). This situation can result from using the ethyl alcohol, which is a main component of the salicylic alcohol, in a diluted form. The grown bacterial colonies can also be an effect of the presence of endospore forms in the palm epidermis, which were not killed by this disinfectant, as the salicylic alcohol does not have a disinfecting effect against the endospore forms of bacteria. The achieved results confirm the thesis of a weak germicidal effectiveness of salicylic alcohol.
In case the bacterial suspension is mixed with oxidized water, the number of bacterial colonies is very low, which demonstrates a good germicidal effect. These results are inconsistent with previous reports [4], in which a 3% solution of oxidized water is described as a weak disinfecting agent. However, it is mentioned that it is used for disinfection of skin injuries. Thus, its germicidal effect on the palm epidermis is weaker than in the bacterial suspension of physiological saline. The high germicidal effectiveness could have been caused by a lack of bacterial catalase in the suspension, which was present in tissues (it causes fast decomposition of hydrogen peroxide, together with a rapid foam formation). Such a reaction in tissues causes a beneficial mechanical action (surging gas bubbles clean the injury mechanically); however, the effect provided by this compound lasts for a very short time (as a result of fast decomposition).
The germicidal effect of iodine tincture amounts to 100%. In the samples treated with iodine tincture, the increase of bacteria number was not demonstrated. According to the bibliography [4, 8] it is an effective and a fast acting compound. It is used for disinfection of injuries? surroundings (it has an irritating effect when applied on the injured skin) and for skin disinfection before entering a sterile surgery room. Iodine tincture kills all forms of bacteria. Its fungicidal and virucidal properties guarantee full sterility. The conducted studies proved these characteristics.
Conclusions
In the conducted experiment, the germicidal effects of disinfectants were compared and it was demonstrated that iodine tincture has the strongest effect, whereas a 0.05% solution of potassium permanganate ? the weakest one. A surprising result was achieved for a 2% solution of salicylic alcohol. Its weak germicidal effect was demonstrated, which was probably caused by excessive dilution in the sample (1:1 with physiological saline). High germicidal effect obtained for a 3% solution of oxidised water could have been caused by conducting the experiment in a test tube rather than in contact with the skin (in vivo conditions). The lack of catalase, which is present in tissues, resulted in that oxidised water was not decomposed in a fast way, which enabled its longer and more effective action.
Literature
1. Bridier A., Briandet R., Thomas V., Dubois-Brissonnet F.: Comparative biocidal activity of peracetic acid, benzalkonium chloride and ortho-phthalaldehyde on 77 bacterial strains Journal of Hospital Infection 2011, 78, 208-213.
2. Hyperlink: www.kosmetyka-krakow.pl, access date: 04.11.2011.
3. Jabłoński L.: Podstawy mikrobiologii lekarskiej. PZWL, Warszawa, 1986.
4. Kostowski W., Herman Z.: Farmakologia. Podstawy farmakoterapii. PZWL, Warszawa, 2004.
5. Lachenmeier D.W.: Safety evaluation of topical applications of ethanol on the skin and inside the oral cavity. Journal of Occupational Medicine and Toxicology, 2008, 3:26,1-16.
6. McDonnell G, Russell AD.: Antiseptics and disinfectants: activity, action, and resistance. Clinical Microbiology Review 1999, 12, 147-179.
7. Mierzejewski J.: Elementy dermatologii kosmetycznej. Wyd. Politechniki Radomskiej, Radom, 2008.
8. Mutschler E.: Farmakologia i toksykologia. Wyd. MedPharm, Wrocław, 2010.
9. Pendlington R.U., Whittle E., Robinson J.A., Howes D.: Fate of ethanol topically applied to skin. Food Chemistry and Toxicology 2001, 39, 169-174.
10. Szewczyk E.: Diagnostyka bakteriologiczna. PWN, Warszawa, 2005.
11. Różalski A.: Ćwiczenia z mikrobiologii ogólnej ? skrypt dla studentów biologii. Wyd. Uniwersytetu Łódzkiego, Łódź, 1996.
12. Zejc A., Gorczyca M.: Chemia leków. PZWL, Warszawa, 2002.
13. Ziembińska A., Duda M., Muc-Wierzgoń M.: A comparison of bacteriocidal influence of disinfectants usedin beauty salons. Polish Journal of Cosmetology, 2011, 14:2, 194-1
Aleksandra ZIEMBIŃSKA ? Ph.D. in biology, microbiologist, works as assistant professor, lecturer in the Department of Environmental Biotechnology of the Faculty of Power and Environmental Engineering at the Silesian University of Technology.
e-mail: ; phone: 94
Marta SZPINDOR ? Eng., a first-level graduate in the field of biotechnology at the Silesian University of Technology. Currently a graduate student in the Department of Environmental Biotechnology of the Faculty of Power and Environmental Engineering at the Silesian University of Technology.
