Małgorzata JAWORSKA, Otmar VOGT ? Institute of Chemistry and Organic Technology, Cracow University of Technology, Cracow, Poland
Abstract:
Gels are the substances with great significance in food industry, pharmaceuticals and cosmetics production as well as in construction chemistry. To form an appropriate consistence of chemical reparations is extremely important for their application properties. A significant difficulty in obtaining gel structure lies in the diversified properties (hydrophobic and hydrophilic) of the ngredients in the preparation. Thus the role of the gelling agent is to maintain, at the same time, these both components. The present paper discusses the methods of preparation of sorbitol and ellulose derivatives. It also proves that these two are effective agents meeting the above requirements.
Please cite as: CHEMIK 2013, 67, 3, 242-249
Introduction
Thickeners and gelling agents in the mixtures of organic solvents and water solutions are widely applied in many branches of industry thanks to their ability to provide the products with the desired utility features e.g. consistency, viscosity or adhesion. The selection of the substance which can guarantee the intended effect depends, first of all, on the character of the ingredients contained in the final product. The properties resulting directly from the chemical structure of the substance used are connected with the strength of interaction with other ingredients of the mixture and they are responsible for the creation and maintenance of the intended product consistence. This property becomes especially significant in the case of mixtures containing the ingredients which, to a large degree, do not blend with each other, and which are of both hydrophilic and hydrophobic type.
A situation in which the ingredients vary so much from one another as far as their character is concerned, is frequent in the composition of cosmetic and household chemical products. Among other situations, this feature often concerns the preparations used for the removal of varnish coats. In the described systems, an appropriate form of the preparation may be obtained when sorbitol derivatives [1, 2] or cellulose derivatives are applied [3,4]. In the case of sorbitol derivatives, some beneficial properties are manifested by 1,3:2,4-di-O- benzylidene-D-sorbitol (D-DBS) in connection with organic solvents. The structure of gels obtained with this substance, varies depending on the polarity of the solvent used. This structure also depends on the solvent?s concentration in the system [5÷7]. Cellulose derivatives, in turn, create hydrogels in water solutions and systems containing sufficient quantity of the water phase. The gels obtained in such preparations are stable in the pH scope from 4 to 10. A characteristic property of such systems is that the presence of polyvalent cations enables cross-linkage and the creation of permanent gels [3].
Gels types and structure
A gel is a system consisting of at least two ingredients, in which each of them creates a separate continuous phase, dispersing in the entire volume of the mixture. Gelling agent creates a stiff, branched, porous net propagating in the liquid, being the other gel component, causing its immobilisation. From the chemical point of view, the gels may be formed by various systems. A liquid phase is frequently water (aquagels), but this phase can also be alcohol or any other organic solvent (organogels). This phase may remain within the system or may be removed within the process of gel drying, leading to the creation of aerogels. The general division of gels is based on the type of reactions responsible for the creation of a stiff network, i.e. a type of gelling agent. There are chemical gels in which, during the process of gel forming, the molecules of gelling agent create covalent bonds between each other, but there are also physical gels in which the molecules of gelling agents are bonded with much weaker intermolecular interactions. Chemical gels comprise, among others, cross-linked polymers, silica gels and gels based on metal oxides. Forming chemical gels is an irreversible process. Physical gels may be formed by very varied substances: both by large protein molecules and also by carbohydrate molecules comprising a few atoms [8, 9].
Gelling substances with a low-molecular weight are characterised by the presence of many functional groups capable of physical interactions, such as van der Waals forces, hydrogen bonds, electrostatic forces, ?-? interactions (?stacking?) and London dispersion forces ? LDF. The process of gelling is based on the formation of fibrous network via interactions between the low-molecular compounds. Then, the organic solvent is adsorbed and trapped in created network [10].
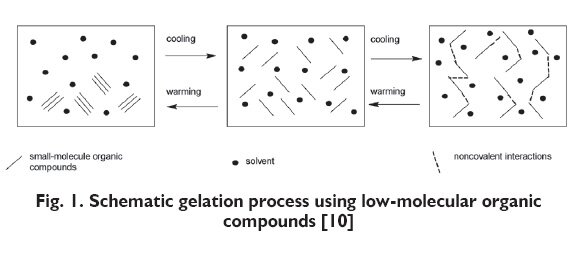
The process of solution bonding or dispersion forming by polymers consists in the addition of water molecules to the macromolecule chain with hydrogen bonds. Polymer chain, however, are connected with each other with permanent covalent bonds [11]. This process takes place in water environment and causes an increase of the effective quantity of molecules, limiting their natural tendencies to coil into compact and solid shapes. The molecules may bond with each other in a linear way with transverse bonds and thus form strong gels, but also branched molecules may bond with each other only in few places [12].
Types of gelling substances
Organic substances with a low molecular weight with the capacity of forming physical gels with organic solvents as continuous phase are called gelators. As a result of this process thermo-reversible gels ? ?organogels? are formed: these are the gels in which the dispersion medium is an organic liquid. Gelators comprise sorbitol derivatives with the units of p-nitrophenol, p-aminophenol and DBS [8]. However, the in the case of cellulose derivatives (hydroxyethylocellulose, methylcellulose, hydroxypropylmethylcellulose, etc.) in water solutions and systems with sufficient quantity of liquid phase, hydrogels are formed. These are systems, consisting of a three-dimensional net of polymer chains and water, which fills the areas between the macromolecules [13].
D-DBS
D-DBS (1,3:2,4-di-O-benzylidene-D-sorbitol) is a derivative of natural sugar alcohol, D-glucitol (sorbitol).
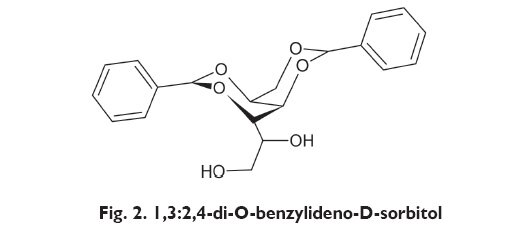
Its synthesis proceeds by means of the condensation of benzaldehyde and sorbitol. In its pure form, D-DBS is a crystalline solid body, with melting temperature of approx. 220oC. It has amphiphilic properties. However, thanks to its hydrophobic phenyl rings, it can dissolve in many organic compounds [8]. It belongs to a group of LMOGs, i.e. low-molecular-mass organic gelators having the ability to organise their molecules in three-dimensional network of nanofibres and, as a result, to gel a wide variety of organic solvents [14]. D-DBS has the ability to gel such solvents as: ethylene glycol, propylene glycol, 1,4-dioxane, glycerol, DMF ? dimethyl formamide, benzene, xylene, hexane, cyclohexane, methanol, ethanol, propanol (propyl alcohol), butanol (butyl alcohol) and others (1). Moreover, it may also form stable gels with many macromolecules (polymers) ? among others with polypropylene (PPG), polyethylene (PEG), silicones and polydimethylsiloxanes (PDMS) [8,14]. The structure of gels formed with D-DBS differs depending on the polarity of the solvent [5÷7]. Solvents with low polarity form weak gels; those with average polarity ? form isotopic structures of metaphase; whilst high polarity solvents form spherulitic (globular) gels.
The methods of D-DBS synthesis are generally known [15÷19]. Among others it is obtained by means of the reaction of benzoic aldehyde and sorbitol in the presence of acidic catalyst (Fig 3).

The reaction of sorbitol with benzoic aldehyde leads to the formation of monobenzylidene sorbitol (MBS), dibenzylidene sorbitol (DBS) and tribenzylidene sorbitol (TBS) [1].
In the method described by Gardlik et al., in the first stage of the process, all the chemical ingredients must be mixed with each other (D-sorbitol, aromatic aldehyde, catalyst and organic solvent). These ingredients may be mixed altogether at once or in groups e.g. sorbitol with alcohol and then with the aldehyde and catalyst. The authors suggest that the solvent should be some alcohol (methanol, ethanol, isopropanol) in such quantity that can make total dissolution of sorbitol possible (the ratio: alcohol/sorbitol = 2.5:1?3:1). For the method, benzaldehyde derivatives containing substituents such as fluorine, chlorine or bromine in the position meta in relation to the carbonyl group can be used. The authors recommend that aldehyde should be added to the reaction mixture in the mole ratio to sorbitol from approx. 1.5:1 to approx. 1.9:1. Arylsulfonic acid (p-toluenesulfonic acid, benzenesulfonic acid, 5-sulfosalicylic acid, naftalenesulfonic acid and their mixtures) can be the catalyst in the above reaction. In the case of p-toluenesulfonic acid, the initial mole ratio between the catalyst and aldehyde should be 0.6:1 ? 0.7:1 [15]. The process can last even up to 48 hours. The product is isolated from the post-reaction mixture by means of extraction with methanol. The consecutive filtrations and extractions are repeated up to 6 times in order to get rid of the catalyst from the system. In the case that more than 0.1% of the catalyst remains in the mixture, DBS is converted to other compounds which will not allow the appropriate gel structure to be formed. This method makes it possible to reduce the quantity of MBS and TBS in the product and also it eliminates water, thus eliminating the necessity to dry the product.
A different method of D-DBS synthesis was suggested by Xie [16]. It differs from the previous one mainly by the application of different catalysts and solvents, which, in the opinion of the authors, is meant to increase the process efficiency and to limit the difficulties connected with the isolation of the final product. The following substances are necessary in order to obtain D-DBS: substituted or non-substituted benzaldehyde, multi-hydroxyoxide alcohol, at least one organic solvent mixing with water and at least one acid catalyst. The catalyst proposed by Xie can be protonic acids (e.g. hydrochloric acid, sulphuric acid, phosphoric acid), Lewis acids or their mixtures. The Lewis acids which are most often used as catalysts are: AlCl3, ZnCl2, SnCl2, SnCl4, SnBr2, SnBr4, MgBr2, FeCl3, BF3. The ratio between the catalyst and benzaldehyde is 0.6:1?0.15:1. The organic solvents mixing with water which are beneficial for this process are: C1-C10 alcohols, acetonitrile, tetrahydrofuran (THF), dioxane or their mixtures. The reaction progresses in an ambient temperature [16].
Scrivens et al. [17] proposed running the synthesis for 2-16 hours in a hydrophobic solvent (e.g.. benzene, toluene, xylene, hexane, cyclohexane), with the concentration of reactants ranging from 5 to 30 %. The temperature of the process depends on the solvent used and varies between 40 and 200°C, with cyclohexane, the process goes on in 75 ? 81°C. The authors proposed Brönsted acids (e.g. HCl, H2SO4, H3PO4, m- and p- toluenesulfonic acid, naftalenesulfonic acid) as the catalysts of the process with the quantity of 0.05?10%. The catalyst was added gradually after warming the reactant to the reaction temperature, either in a form of a pure acid or as a solution in the solvent used for the reaction. After the process it was necessary to neutralise the reaction mixture with NaOH solution and repeatedly rinse it with water.
Uchijama [18] carried out the syntheses in cyclohexane, maintain the ration between cyclohexane and benzaldehyde as 2:1. In the presence of water, in these condition azeotropic mixture is formed and its boiling point is approx. 68.95°C, which determines the temperature of the entire process. In the author?s opinion, the necessary conditions for the formation of the appropriate diacetyl is to maintain the proposed ratio between benzaldehyde and sorbitol = 2:1 and also to follow closely the requirements concerning the time scale for running the process (from 5 to 7 hours ). The prolongation of the reaction time has an adverse influence on the process efficiency. The author suggests alternative solvents such as hexane, N,N-dimethyl formamide, dimethyl sulfoxide, dioxane, methanol, ethanol, propanol and butanol. For the process, acid catalyst is used (e.g. 35 % HCl, concentrated H2SO4, 80-90 % H3PO4 ) with the quantity from 0.1 to 10 %. Similarly, Machell [19] proposes to carry out the synthesis in cyclohexane, in the temperature of about 70°C in the presence of acid catalysts. The author states, however, that the ratio benzaldehyde : sorbitol from 1:0.75 to 1:1.75 is advantageous for the process.
Gels formed by D-DBS
The structure of the gels formed by D-DBS depends strictly on the polarity of the solvent matrix and on the concentration of the gelator in the system. The research carried out by Yamasaki and Tsutsumi [7] showed that the form of the gel formed by D-DBS depends on its concentration in the system which is treated by the gelling process. The experiments were carried out in the following system: D-DBS/ethylene glycol. It was found that the gelling process occurs with the concentration above 10 mmol/dm3, and the concentration between 10 and 30 mmol/dm3 shall guarantee its highest efficiency. With higher concentrations, crystals precipitate in the entire gel structure.
Moreover, polarity degree of the solvents mixture has a strong influence on the intermolecular hydrogen bonds which are formed during the process of gelling. The more polar matrix, the larger advantage of the hydrogen bonds formed between the solvent and DBS over the DBS-DBS bonds. Yamasaki and Tsutsumi [5] proved that as a result of mixing DBS with non-polar p-xylene, a net consisting of spiral nano-fibres with the diameter of approx. 100 nm, is formed. In the case of medium-polar matrix, such as 1,4-dioxane, DBS forms a structure in with there is an isotopic mesophase (liquid crystal phase). However, in the case of more polar solvents, such as for example dimethyl formamide, the gels have spherical structure. In the case of DBS gels formation, in ethylene glycol, a two-phase system is formed, consisting of the spherical structure areas, being the crystalline phase and mesophase between the spherulitic (globular) areas [6]. The crystalline phase consists of DBS molecules organised in spiral (helical) aggregates. However, the isotropic mesophase ahs a network structure similar to the structure of DBS molecules. It has the ability of shifting into the crystalline phase depending on the temperature [6].

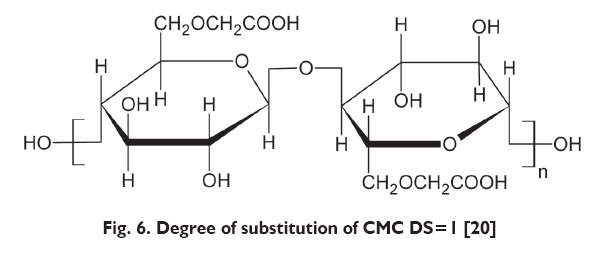
Cellulose is a polysaccharide consisting of D-glucose units connected 1,4-?-glycoside bonds. Thanks to this, cellulose forms fibres consisting of a few thousand glucose radicals connected with one another in a linear aliphatic (open chain) biopolymer. Specific chains are lying close to each other creating in the majority of cases, crystalline areas, but in the areas where the chains are not arranged in a parallel way to one another, amorphic structures are formed. With regards to the presence of numerous hydrogen bonds between the chain, cellulose is not water soluble, even in hot temperature. The number of D-glucose units in the cellulose polymer chain is defined as Degree of Polymerization ? DP. Moreover, in each D-glucose ring there are three chemically active hydroxyl OH formations, in the following positions: 2, 3 and 6. In those places it is possible to introduce another function group (methyl, ethyl, carboxymethyl, propylene oxide, acetate or nitro group). The number of OH groups substituted with another group in each D-glucose unit is called the Degree of Substitution ? DS) [4, 20]. The easiness with which cellulose forms its derivatives makes it an attractive raw product (ingredient), allowing for adapting the structure to the planned properties [21].
The influence of the introduced groups on the properties of the modified cellulose depend on the type of the substituent [20]. The degree of substitution (DS) influences the degree of swelling and water solubility of cellulose derivatives. In the case of CMC, the derivatives with less than 1/3 of hydroxyl groups substituted, dissolve only in alkalies; derivatives with DS index =0.6 dissolve both in cold and in warm water. Modified CMC contains numerous anionic units. It forms hydrogels and is insensitive to the presence of single-valued or bivalent cations [20].
Cellulose ethers and their derivatives
Methylcellulose is a customary name of cellulose ethers, such as methylcellulose (MC), methyl hydroxy ethyl cellulose (MHEC), hydroxypropyl methyl cellulose (MHPC), hydroxyethyl cellulose (HEC), hydroxypropyl cellulose (HPC), carboxyl methyl cellulose (CMC) and a few other cellulose derivatives [22].
One of the main methods of ether preparation is Williamson?s reaction (Fig. 5). Cellulose etherification is carried out in alkaline conditions, usually with the use of sodium hydroxide. Cellulose is first treated with NaOH solution, forming alkalicellulose which reacts with etherifying agent [23].
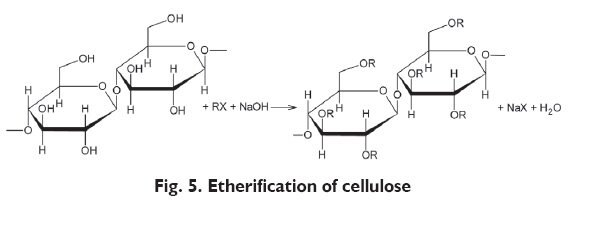
In industry, the following substances are used as RX etherifying agents: methyl chloride, ethyl or other hydrogen chlorides in the presence of auxiliary substances. As a result of the above processes, hydrogen atoms of hydroxyl groups in the particles of insoluble cellulose are substituted with methyl, hydroxyethyl, hydroxypropyl or other groups [22].
Other methods of ether preparation are cellulose addition reactions to the following:
? epoxy ring and then cellulose is catalysed with RX acid, where R=H, -CH3, -C2H5,
? active double bond, and then the reaction is carried out in alkaline environment (in this reaction, a substituent which strongly attracts electrons is attached to carbon with double bond, such as -CN, -CONH2 or -SO3Na group [23].
From the patent overview described in the article of Szczygielska and Kijeński [24] it seems that further cellulose ether modifications may lead to forming thickeners with refined rheological properties. Cellulose ethers used for this type of modification are its methyl, hydroxyethyl or hydroxypropyl derivatives. The hydrophobising compounds used in this process are glycidyl ethers, haloids, halogenated hydrines, esters, acid anhydrides, arylated haloids and other compounds with hydrophobic chain with function group capable of reacting with -OH groups [22, 24].
CMC forms stable-plastic viscous solutions which stabilise suspensions well, within the pH range from 4 to 10. The ability to stabilise suspensions increases together with DP increase and decreases with DS. An advantage of CMC is its ability to bond water in the quantity a few times larger than the mass of dry gelator [20]. Hydroxypropyl methyl cellulose (MHPC) has a form of white or cream-white powder and is easily soluble in cool water. MHPC almost does not dissolve in organic solvents such as: ethanol, ethers, acetone. It has membrane-forming, densifying, dampening, dispergating and emulgating properties. Its water solutions are pseudo-plastic, they are transparent, thermolabile and insensitive to the presence of electrolytes and their tolerance increases together with an increase of the contents of units with propylene oxide. They are stable in the pH range from 3.5 to 11 [4, 20].

Gels formed by cellulose derivatives
Hydrogels consist from a three-dimensional net of polymer chains and water, which fill the areas between macro-molecules [13]. In a dry state they have a tendency to form tightly convoluted, compact coils. Attaching water molecules to the polymer limits this tendency causing an increase of the effective dimension of the molecules. The larger molecular weight of hydrocolloid and the number of function groups and side chains, the larger the possibility of binding water by the extended molecules of this hydrocolloid and this, in turn, causes larger viscosity of the water solution [25]. Once water is added, the polymer function groups are surrounded by liquid, as a result of which dissociation occurs. Cations separate, whilst negatively charged polymer radicals repel each other as a result of electrostatic forces. Free spaces are created between the chains and water can penetrate into them. The process lasts until the moment of maximum elongation of polymer chains [26]. Spatial net of hydrogels depend on physical and chemical properties of the polymer. In the case of cellulose derivatives, this net may assume the form of an entangled helix [13].
Recapitulation
Gels may be, among others, drug and active substance carriers in cosmetics; they may also be the ingredients of adhesive mortars and preparations for the removal of varnish coats. Their appropriate consistency is guaranteed by the selection of an adequate gelling substance. Among numerous substances modifying rheological properties, a significant role is played by D-DBS and cellulose derivatives, such as MHPC. Each of these densifiers forms a different gel type. Sorbitol derivatives belong to low-molecular substances causing the formation of thermo-reversible ?ogranogels? as a result of physical reaction. Cellulose and its derivatives, in turn, as bio-polymers, cross-link the ?hydrogels?, formed as a result of both chemical and physical reactions. The discussion of the research carried on the application of D-DBS and MHPC in complex preparations designated for the removal of varnish coats is presented in a separate article.
Literature
1. Tomaszkiewicz-Potępa A., Sikora E., Heród D.: Zastosowanie dibenzylidenosorbitolu jako substancji zagęszczającej. Chemik 2005, 58, 11.
2. Sikora E., Tomaszkiewicz-Potępa A., Rączka K.: Małocząsteczkowe substancje zagęszczające. Chemik 2005, 10, 531.
3. Sikora M.: Modyfikatory reologii, środki zagęszczające i żelujące. Rynek kosmetyków i farmaceutyków 2007, 2, 36.
4. Karta charakterystyki: Modyfikator reologii Culminal MHPC 20000 S. Hercules International Ltd. Netherland 16.08.2007.
5. Yamasaki S., Tsutsumi H.: The Dependence of the Polarity of Solvents on 1,3 : 2,4-Di-O-benzylidene-D-sorbitol Gel. Bull. Chem. Soc. Jpn. 1995, 68, 1, 123.
6. Yamasaki S., Tsutsumi H.: Microscopic Studies of 1,3 : 2,4-Di-O-benzylidene-D-sorbitol in Ethylene Glycol. Bull. Chem. Soc. Jpn. 1994, 67, 4, 906.
7. Yamasaki S., Tsutsumi H.: The Phase Transition in the Gel State of the 1,3 : 2,4-Di-O-benzylidene-D-sorbitol/Ethylene Glycol System. Bull. Chem. Soc. Jpn. 1994, 67, 8, 2053.
8. Wilder E., Braunfeld M., Jinnai H., Hall C., Agard D., Spontak R.: Nanofibrillar Networks in Poly(ethyl methacrylate) and Its Silica Nanocomposites. J. Phys. Chem. B 2003, 107, 42, 11633.
9. Luboradzki R.: Substancje na granicy. Academia 2009, 20, 4, 36.
10. Shibata M.: Soybean ? Molecular Aspects of Breeding. InTech 2011, 473.
11. Tyliszczak B., Pielichowski K.: Charakterystyka matryc hydrożelowych ? zastosowanie biomedyczne superabsorbentów polimerowych. Czasopismo techniczne 2007, 1-Ch, 159.
12. HYPERLINK ?http://www.up.poznan.pl/ztoiw/dydaktyka/zelowanie.pdf? Materiały dydaktyczne Zakładu Technologii Owoców i Warzyw Uniwersytetu Przyrodniczego w Poznaniu 28.11.2012.
13. Sosnowska K.: Hydrożele jako nowoczesna postać leku. Gazeta Farmaceutyczna 2009, 2, 34.
14. Wilder E., Antonucci J.: Improved Dental Composites Utilizing Dibenzylidene Sorbitol Networks. Macromolecular Sympozia 2005, 277, 1, 255.
15. patent nr US 5,106,999, USA.
16. patent nr US 2006/0079720, USA.
17. patent nr US 5,731,474, USA.
18. patent nr US 4,267,110, USA.
19. patent nr US 4,562,265, USA.
20. Fabianowski W.: Modyfikatory reologii pochodzenia naturalnego. Wiadomości PTK 2001, 4, 3-4, 42.
21. Nishinari K., Takahashi R.: Interaction in polysaccharide solutions and gels. Current Opinion in Colloids and Interface Science 2003, 8, 4-5, 396.
22. HYPERLINK ?http://www.izolacje.com.pl/artykul/id439,skladniki-zapraw- klejowych-do-plytek-cz-ii-metyloceluloza? Miesięcznik Izolacje 20.11.2012.
23. HYPERLINK ?http://www.polsl.pl/Wydzialy/RCh/RCh2/Documents/ zwnatCHceluloza.pdf? Materiały dydaktyczne Katedry Chemii Organicznej, Bioorganicznej i Biotechnologii Politechniki Śląskiej ?Otrzymywanie karboksymetylocelulozy? 15.09.2012.
24. HYPERLINK ?http://www.chemical.pl/artykuly/chemical-review/6456/ modyfikowane-etery-celulozy-8211-cenne-surowce-w-nowoczesnych- zastosowaniach.html? Chemical Review 28.11.2012.
25. HYPERLINK ?http://www.czytelniamedyczna.pl/3228,hydrokoloidypochodzenia- roslinnego-jako-zamienniki-zelatyny.html? Bezpieczna żywność 28.11.2012.
26. Łukasik N., Kamińska B.: Hydrożele. Chemik Light 2012, 6, 12.
Małgorzata Jaworska ? M.Sc., graduated from the Faculty of Chemical Engineering and Technology Tadeusz Kościuszko Cracow University of Technology in 2008. Currently she is a doctoral student in the Institute of Chemistry and Organic Technology at the Faculty of Chemical Engineering and Technology of Tadeusz Kościuszko Cracow University of Technology. She specialises in cosmetics chemistry and technology, in particular in forming nano-emulsions and studying their properties.
e-mail: ,
tel.:
Otmar Vogt ? Ph.D., graduated from the Faculty of Chemical Engineering and Technology of Tadeusz Kościuszko Cracow University of Technology in 1990. He obtained the doctoral degree in chemical sciences at the Faculty of Chemistry of the Jagiellonian University. He is an associate professor in the Chair of Organic Technology and Refinery Processes at Tadeusz Kościuszko Cracow University of Technology. Specialization ? catalysis, organic technology, fine chemicals technology, including the research on forming natural-origin substances and their application in environmental friendly household chemicals and natural pesticides.
e-mail: ,
tel.:
