Marek ŚCIĄŻKO ? Institute for Chemical Processing of Coal, Zabrze – Leszek STĘPIEŃ ? AGH University of Science and Technology, Krakow, Poland
Please cite as: CHEMIK 2013, 67, 5, 379?386
Introduction
Solid fuels, including primarily hard coal and lignite originated as a result of physicochemical conversion of primary organic substance under the influence of temperature, pressure and in a basically anaerobic environment. This process, referred to as a metamorphism phase, was accompanied by, inter alia: evolution of carbon dioxide, water and methane. The higher conversion degree was achieved by the original organic substance, the more ordered was coal structure and increasingly larger domains of condensed aromatic structures. Ultimately the fuel properties are decided by the temperature-time history of conversion, manifesting itself in the elemental composition expressed mainly by the C, H, O, N, and S content and additionally by the ash content. To determine technological usability of coal additional physicochemical analyses are sometimes performed, such as: plasticity and caking ability, when coal is used in the coke making, and volatiles, ash characteristic temperatures or the calorific value in the case of power generation applications. Basically, results of performed tests refer to a specific sample of coal and unfortunately they cannot be catalogued like in the case of pure, structurally defined chemical compounds. The thermodynamic potential of a substance is defined by values of standard normal functions, including primarily the enthalpy of formation, which reflects energy effects accompanying the conversion. In the case of chemically unidentified structures, like in coal, of varying composition, determination of the enthalpy of formation requires slightly different testing procedures than in the case of pure chemical substances. The knowledge of the enthalpy of formation value of coal organic substance allows its comparison with others, but it is also necessary for proper balancing of coal thermal conversions. The coal structure formed in a peculiar georeactor has specific features characterising the susceptibility to further conversions. In its nature, the geochemical coalification of the original organic matter is an inhibited pyrolysis and all technological directions of coal utilisation have a nature of thermal processes that is during the first stage they are related to its pyrolysis, in this case being a controlled and accelerated continuation of reactions proceeding in the coal deposit. From this point of view the defining of coal kinetic parameters may reflect the effect of its geochemical conversions, taking into account characteristic parameters of the chemical composition, and also allows determining this technological usefulness. In particular, kinetic parameters of coal will characterise its generally understood reactivity. However, in this case we encounter an additional difficulty related to the influence of coal heating rate on the course of thermal decomposition curves, which is typical of reactions proceeding in a heterogeneous system. To compare various coal fuels and in particular their behaviour in processes of thermal conversion it is necessary to find a method for determination of basic kinetic parameters taking into account the heating rate variability and the lack of knowledge about the course of elementary reactions. Possessing the information on both the enthalpy of various coals formation and on kinetic parameters it is possible to determine not only the state of organic substance metamorphic conversions, but also to classify coals for technological purposes [1]. So the paper suggests a method for determination of the enthalpy of coal formation and of its kinetic parameters, which are subsequently used for their classification reflecting their structure and technological usability. The coal classification suggested from a thermodynamic perspective has a nature of absolute scale, while the values of kinetic parameters of coals analysed are right for the adopted pyrolysis model and therefore the kinetic classification is relative.
Enthalpy of formation
The enthalpy of formation represents the energy result of the course of reactions, which resulted in the considered product formation. For pure substances the enthalpy of formation assumes one defined value. Instead, because of a different chemical structure of coal the enthalpy of formation to a large extent will depend on the rank of coal and on the structure specific for a given coal. The balancing of coal conversion processes may be carried out based on the knowledge of the calorific value of coal and of its conversion products or based on the method utilising the enthalpy of formation used in chemical thermodynamics. The latter method provides also grounds for commercial computer codes used for process computations (EES, ChemCad, Aspen) [2, 3, 4]. Unfortunately, in the case of coal in those codes it is assumed that it is a physical mixture of pure elements and because of that the enthalpy of coal formation equals zero. Such an approach is the source of major energy balance errors.
Thermal Effect of Reaction and Enthalpy of Formation
Calorimetrically determined heat of combustion of coal differs from the sum of the enthalpy of combustion of the elements contained in the coal substance being in the standard state. The difference existing between these values is treated as a definition of the enthalpy of coal formation. A negative value of the enthalpy of formation means that the creation of specific organic structure CxHyOz caused a release of defined amount of heat. Hence to bring this organic substance to decomposition it is necessary to deliver an equivalent heat, which should be considered in the energy balance of the considered coal conversion process. Different values of enthalpy of formation result basically from the difference in the structure of coal organic substance. Taking into account Polish coals classification [5] and a typical elemental composition of the organic matter of coals quoted by Jasieńko after Roga [6], it is possible to analyse a change of the atomic composition with increasing degree of coalification taking into account three basic elements: C, H, and O, which is presented in Figure 1. It is possible to notice that the greatest changes are related to the involvement of oxygen atoms.
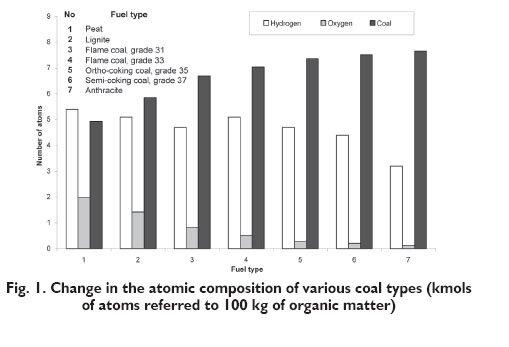
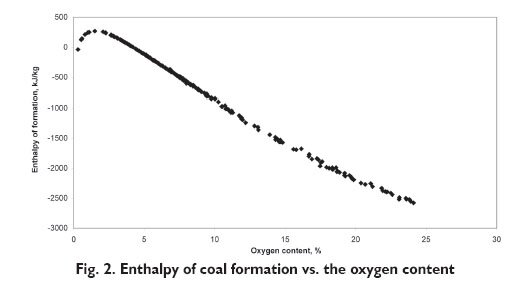
This fact was confirmed by results of experimental studies, in which 224 coal samples, originating from Russian [7], American [8] and Polish [9] mines, were analysed. The enthalpy of formation dependence on the oxygen fraction was determined based on these results, which is presented in Figure 2. The regions of positive value can be distinguished for the oxygen content below 4% and positive above.
The applied method for determination of the enthalpy of coal formation consists in determination of the difference between the enthalpy of coal component elements, being in standard state, combustion reaction and calorimetrically determined heat of combustion [10], which is expressed by equation (1).

This method gives the same result in the case of elemental carbon, and more precisely of its allotropic form of hexagonal graphite, for which the standard enthalpy of formation is equal to zero. Thermodynamically determined enthalpy of elements combustion reaction ?cH0 is described by relationship (2). The numerical coefficients in this equation were calculated based on standard enthalpy of the combustion of elements (C, H, S), and element fractions are given in percentages of dry matter:
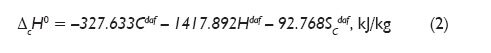
The difference between the calorimetric heat of coal combustion (Qs>0) and the enthalpy of combustion of the constituents (DcH0 <0) depends on the structure and elemental composition of coal. It is therefore desirable to derive a correlation between the heat of combustion and the enthalpy of the combustion reaction of coal constituents. In general, such relationship may be expressed in the form of the following equation:

where: f(?) is the function correcting the enthalpy of combustion reaction which expresses the ratio of the absolute values of combustion heat and of the enthalpy of the combustion reaction of the constituents.
Based on the analysis of the effect of the individual elements on the heat of combustion, an assumption was made that the correcting function f(?) depends on the oxygen concentration in coal Od daf. As this is an empirical function, it is expressed in the form of a power series.
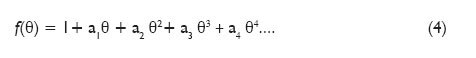
where ? is the oxygen content expressed in mass percentage.
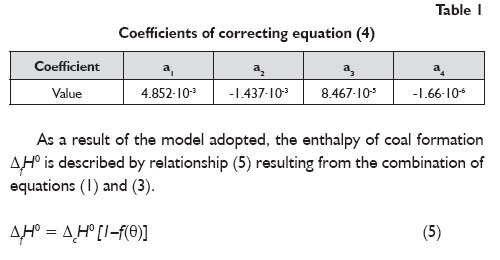
The constant coefficients in equation (4) were determined by correlating experimental data; the results obtained are listed in Table 1.
The enthalpy of formation for any coal fuel may be calculated after determination of elemental composition and then calculation of the correcting coefficient described by the oxygen content function. Calculations should be carried out for dry ash-free state. The results obtained, if necessary, may be transposed to the actual conditions with account taken of water or ash content. When elemental composition of fuel and oxygen content are known, then model (5) enables calculation of the value of standard enthalpy of coal formation. This was the basis for drawing up a new thermodynamic classification of Polish coals, which is shown in Figure 3.
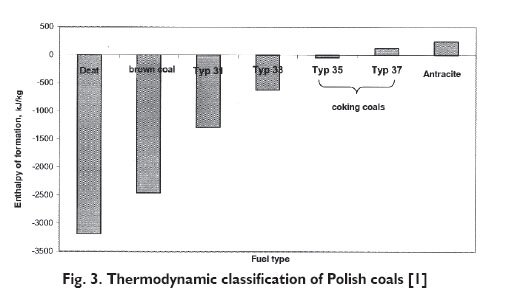
As can be seen, the enthalpy of coking coals formation is nearly zero. The enthalpy of anthracite formation is positive, though very low. All other types of coal have negative enthalpies of formation and the younger the coal the higher the absolute value of formation enthalpy.
Kinetics of Solid Fuels Decomposition Reaction
Coal is a complex solid substance and therefore in kinetic modelling we use a simplified approach, allowing however representing the actual conditions. Thermal conversions of coal with chemical structure difficult to define and texture changing during the conversion make that many model approaches originated so far, taking into account the reaction order, autocatalytic and diffusion effects as well as structural changes [11]. Various points of view on the pyrolysis kinetics may be found in the literature; however, the basis for all analyses consists of a decomposition reaction model, proceeding in accordance with the following notation, presenting as a result an individual irreversible reaction of thermal decomposition of coal:

In the literature this notation is frequently considered oversimplified, because it skips significant intermediate stages of coal decomposition, including primarily the consecutive and possibly parallel nature of the decomposition reaction of primary coal tar (metaplast) postulated already by Van Krevelen [12]. Because of that many forms of kinetic equation have been formulated, dividing also the pyrolysis into individual stages [13]. However, a comparison of various schemes of coal organic matter decomposition course leads to a conclusion that irrespective of the path for final products achieving, the notation expressed by relationship (6) reflects the final result of pyrolysis. As a result, numerous authors approximate the process of coal decomposition by the first order reaction proceeding evenly throughout the particle volume [14, 15]. In such a case the volatiles evolution rate can be described by the relationship:

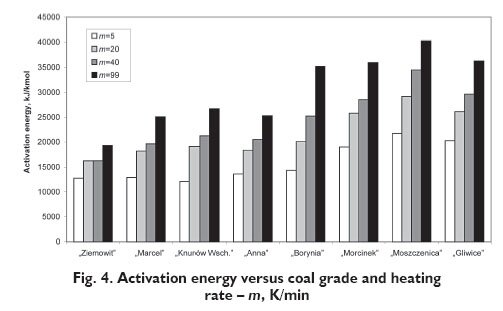
The adoption of the above approach allows determining averaged values of activation energy E and the frequency factor k0 for the studied pyrolysis range. These values were determined based on results of thermogravimetric studies carried out at the Institute for Chemical Processing of Coal. Pyrolysis of coals of various coalification degrees was carried out on a laboratory scale using a LECO TGA- 501 thermogravimetric analyser, using samples weighing 1?5 g. The instrument measures changes of mass at 0.00025 g sensitivity of the balance. The studies were carried out in a nitrogen atmosphere at the heating rates of 5 K/min, 20 K/min, 40 K/min and 99 K/min and at the following final temperatures: 450, 550, 600, 650, 700, 750, 800, 850 and 900°C. Pyrolysis was carried out for coal samples preliminary dried to a constant mass, at the temperature of 105°C [1]. Both the activation energy and the frequency factor were determined using the method of error minimisation between the measured data of mass (volatiles) loss and the model equation. Values of activation energy for various coal types and at various heating rates are presented in Figure 4.
Similar results were obtained for the frequency factor taking into account the influence of heating rate. A detailed analysis allowed finding the relationship between volatiles content in coal, its heating rate and appropriate kinetic parameters. Equations (9) and (10) present appropriate correlations.
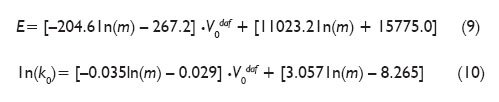
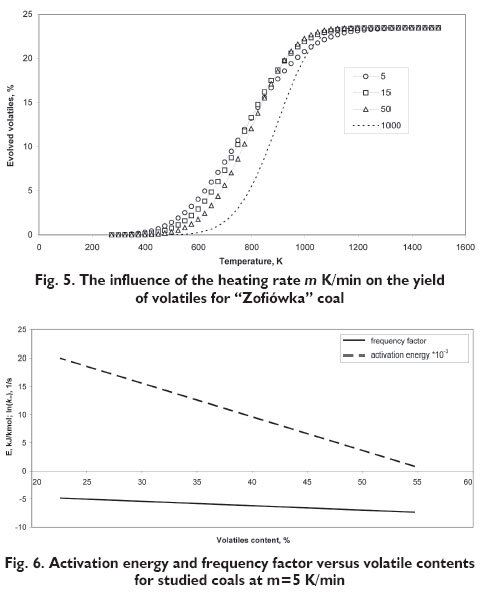
Assuming the above model equations the simulation calculations were performed for the ?Zofiówka? coal for various heating rates m = 5, 15, 50 and extrapolated for 1000 K/min and the results are presented in Figure 5. It is possible to notice that an increase in the heating rate moves appropriate mass loss curves to the right. Because of that to compare kinetic properties of individual coals it is necessary to indicate a reference heating rate. Assuming that this rate is 5 K/min we obtain the relationship between kinetic parameters and volatiles content in coal, presented in Figure 6. So it is possible to use the presented methodology for solid fuels classification.
Conclusions
A general methodology for the calculation of enthalpy of formation and respective kinetic parameters was proposed for fuels characterized by undefined chemical formula. Taking that into account the study resulted in a suggestion for coal classification models from thermodynamic and kinetic perspective, reflecting its structure and technological usability.
A uniform method for determination of the enthalpy of coal formation has been developed, which is related to the heat of combustion and to the enthalpy of combustion reaction of constituent elements of coal, either calculated as the difference between these values or expressed by model (5). As a result of kinetic analysis of experimental data, correlations have been prepared taking into account both the influence of the coal type and of the heating rate on the change of kinetic parameters, i.e. the activation energy and the frequency factor. The results obtained shows that between V0 daf and kinetic parameters E and k0 there are roughly linear relationships. The developed methodology is helpful for comparative studies between various solid fuels of CxHyZy type.
Acknowledgements
Work performed under task of research No. 3 funded under the Agreement NCBiR No. SP/E/3/7708/10 and within AGH in Krakow statutory work No. 11.11.210.213.
Literature
1. Ściążko M.: Modele klasyfikacji węgla w ujęciu termodynamicznym i kinetycznym (Coal Classification Models in a Thermodynamic and Kinetic Approach). Dissertations and Monographs, AGH-Krakow, 2010.
2. http://highered.mcgraw-hill.com/sites/0072383321/information_center_ view0/ees_software.html.
3. http://www.chemstations.com/.
4. http://www.engin.umich.edu/~cre/help/software/html/aspen/index.htm.
5. Jasieńko S.: Chemia i fizyka węgla (Chemistry and Physics of Coal). Publishing House of the Wrocław University of Technology, Wrocław 1995.
6. Roga B., Wnękowska L., Ihnatowicz A.: Chemia węgla (Chemistry of Coal). Warsaw, WNT, 1955, p.237.
7. Gagarin S.G., Gladun T.G.: Ocienka entalpii obrazowania organiczieskoj massy kamiennych ugliej i antracitov. Chemia Tvierdovo Topliwa, 2003,vol.4, p.3.
8. Coal Conversion Systems Technical Data Book, section IA.50.1,vol.2, 1980.
9. Ściążko M et al.: Bilansowanie procesu koksowania węgla typu 35 (Balancing of Coal Type 35 Coking Process). ICHPW Report, 30/2010.
10. Annamalai K., Puri I.K.: Combustion science and engineering. CRC Series, Boca Raton, FL, 2005, p.169.
11. Keii T.: Heterogeneous Kinetics. Sprinter, Heidelberg, 2004.
12. Van Krevelen D.W.: Coal. Elsevier, Amsterdam, 1961.
13. Mianowski A., Radko T.: Thermokinetic analysis of coal pyrolysis process. Journal of Thermal Analysis, 1995, vol.43, p.247.
14. Solomon P.R., Hamblen D.G.: Pyrolysis. Chemistry of Coal Conversion. R.H. Schlosberg (ed.), Plenum Press, New York,1985.
15. Howard J.B.: Fundamentals of Coal Pyrolysis and Hydropyrolysis. Chemistry of Coal Utilization. M.A. Elliott, ed., John Wiley & Sons, New York, 1981.
Marek ŚCIĄŻKO ? Ph.D., (Eng.), Professor, graduated from the Silesian Technical University in 1975. In 1980 he hold fellowship at the Pittsburgh Energy Technology Center, USA, where performed research on modelling of coal gasification, which resulted in his Ph.D. thesis. In 1993 he was awarded a management fellowship at the University of North Dakota, U.SA, in the field of energy efficiency. He was the project manager and the deputy director of Polish-German Research Centre for Coal Gasification 1987÷1993. He holds the position of managing director of Institute for Chemical Processing of Coal since 1991. Member of Advisory Group for Energy ? DG RTD EU, Energy Committee of Polish Academy of Science, Supervising Board of energy group ?TAURON?, professor at the University of Science and Technology (AGH) in Cracow. He is co-author of 119 articles, 29 monographs and 52 patents.
e-mail: ;
phone:
Leszek STĘPIEŃ ? M.Sc., scientific assistant at the University of Science and Technology (AGH) in Cracow.
e-mail: ;
phone:
