Agnieszka WOŁOSIAK-HNAT, Eugeniusz MILCHERT ? Institute of Organic Chemical Technology, West Pomeranian University of Technology, Szczecin, Poland
Please cite as: CHEMIK 2013, 67, 6, 572-579
The comparison of the course of hydrogenolysis performed in protic solvent as a source of hydrogen in situ with hydrogenolysis carried out in the presence of molecular hydrogen was made. As a donor of hydrogen molecule 1- and 2-propanol were used. Hydrogenolyses with hydrogen generated in situ were conducted using two methods: under inert gas pressure (nitrogen) and pressureless ? in the absence of inert gas and molecular hydrogen.
Introduction
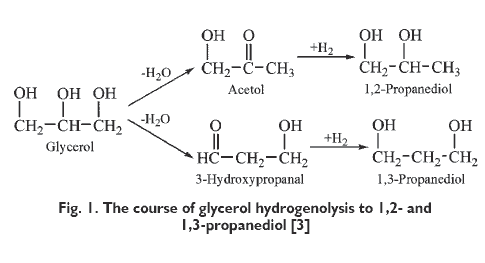
Biodiesel production based on transesterification of vegetable oils with methanol or ethanol provides large amounts of waste glycerol, containing about 30?80 %wt of glycerol [1]. For every 10 kg of biodiesel produced, about 1 kg of waste glycerol is formed [2]. Therefore, there is a need to manage the glycerol contained therein. Firstly, the methods of manufacturing glycerol to more valuable chemical compounds have been sought. One such method is hydrogenolysis of glycerol to 1,2-propanediol. It involves the reaction of glycerol with hydrogen in the presence of heterogeneous catalyst. The reaction proceeds is two steps. The first one involves the glycerol dehydration to an intermediate ? acetol or 3-hydroxypropanal while the second step ? hydrogenation of acetol and 3-hydroxypropanal to 1,2- and 1,3-propanediol, respectively (Fig. 1).
The majority of works concerning glycerol hydrogenolysis process reported the results of hydrogenolyses carried out in aqueous glycerol solution under hydrogen pressure. Due to the fact that water is formed also as a byproduct in hydrogenolysis during acetol or 3-hydroxypropanal formation, introduction of water to reaction mixture may have negative effects on reaction equilibrium in the first step of the reaction [4]. In addition the introduction of hydrogen under pressure is associated with higher demands on the apparatus. A more favorable solution may be to generate hydrogen in situ using solvent as donor proton. This method of carrying out the process is simpler, safer and less expensive. In order to determine the effectiveness of hydrogenolysis carried out with in situ generated hydrogen from solvent dehydrogenation, the hydrogenolyses without molecular hydrogen (B and C) and also with molecular hydrogen (A) were performed. In the case of hydrogenolyses performed with in situ generated hydrogen, experiments were carried out by a pressureless method without molecular hydrogen and by a pressurized method using inert gas atmosphere (nitrogen). In the pressurized method molecular hydrogen was also not introduced. Solvents performing the function of donor molecule were 1- and 2-propanol.
Experimental
Materials
The studies of hydrogenolysis were performed with 98 %wt glycerol from Chempur, Piekary Śląskie, hydrogen 99.99 %wt supplied by Messer and solvents: 1-propanol and 2-propanol ? analytically pure, purchased from POCh SA For catalyst preparation, copper nitrate Cu(NO3)2?3H2O, aluminum nitrate Al(NO3)3?9H2O and potassium carbonate K2CO3 of high purity, obtained from POCh SA were used.
Catalyst preparation
Cu/Al2O3 catalyst was prepared by co-precipitation method based on method described by Mane et. al. [5]. The 0.05 M aqueous solutions of copper nitrate, Cu(NO3)2?3H2O and aluminum nitrate, Al(NO3)3?9H2O were mixed at room temperature, under vigorous stirring and precipitated using 0.2 M aqueous solution of potassium carbonate, K2CO3. The precipitate was aged for 24 h, filtered and washed with deionized water to pH=7. The precipitate was dried at 90 °C for 6 h and calcined at 400°C for 4 h. The catalyst was prereduced under hydrogen at 300°C for 8 h.
Catalyst characterization
Phase composition of Cu/Al2O3 catalyst was determined using X-ray diffraction method. X?ray diffraction measurements were recorded on an X?Pert PRP, Philips diffractometer. This method was also used to evaluate the copper crystalline size in Cu/Al2O3 catalyst based on the Scherrer formula. Morphology of catalyst was studied with transmission electron microscopy using a TEM-FEI Tecnai F20 microscope (200 kV). Nitrogen adsorption and desorption isotherms determined using ASAP 2010 (Micrometrics Instruments) were used to determine the catalyst surface area (BET), its average pore volume and average pore diameter (BJH).
Hydrogenolysis
The hydrogenolysis of glycerol was carried out in a 150 ml capacity autoclave equipped with a PTFE insert. The reactor was charged with 20 ml of glycerol solution of 70 %wt glycerol in solvent and 5 %wt of catalyst in relation to introduced glycerol. In the pressureless method of hydrogenolysis, performed with in situ generated hydrogen, the autoclave was filled several times with nitrogen up to a pressure of 5.0 MPa followed by its reduction to the atmospheric value. In the pressurized method, after the last filling of autoclave with nitrogen ? up to 2.0 MPa, the autoclave was placed in a heating jacket. In hydrogenolysis carried out under hydrogen pressure, after introduction of solvent (1- or 2-propanol) the autoclave was purged several times with hydrogen and filled up with hydrogen up to 2.0 MPa. It was then placed in a heating jacket. Reaction mixture was heated to 200°C. The temperature was measured by a thermocouple connected to a temperature controller. The reaction mixture was stirred with a magnetic stirrer. The stirring speed was 500 rpm. After 24 h reaction time the autoclave was cooled to the ambient temperature and the amount of gas phase was measured using gas meter (Elster-Amco). The gas phase was collected to a gas bag and the samples for analyses were taken into gas pipette. The catalyst was separated from the liquid phase by centrifugation. The solution was decanted and quantitative analyses of products and unreacted glycerol were performed
Analytical methods
Quantitative analyses of liquid phase, consisted mainly of 1,2-propanediol and unreacted glycerol, were carried out by the method of internal standard (phenol) using gas chromatography. The gas chromatograph was equipped with a flame ionization detector and a glass capillary column DB-WAX 30 m × 0.25 mm × 0.5 ?m. The chromatograph was provided with computer data collection and handling software ? the Chrom-Card for Trace GC. The following temperature program was used: 5 minutes isothermal at the temperature of 100°C, increase to 240°C at the rate of 10 °C/min and 6 minutes isothermal at 240°C. The carrier gas (helium) flow was 2 ml/min, hydrogen flow 30 ml/min, air flow 350 ml/min. The detector temperature was 240°C, and sample chamber temperature 100°C. Quantitative analyses of volatile products in gas phase: methanol, ethanol, 1- and 2-propanol and the same products in gas phase including hydrocarbons: methane, ethane and propane were carried out using gas chromatography with absolute calibration. Gas chromatograph was equipped with flame ionization detector and steel column (3 m × 4 mm) filled with Porapak Q. Analyses were performed using the following temperature program: 4 minutes isothermal at the temperature of 100°C, increase to 190°C at the rate of 15 °C/min. The carrier gas (nitrogen) flow was 23 ml/min, hydrogen flow 40 ml/min, air flow 400 ml/min.
Catalyst characterization
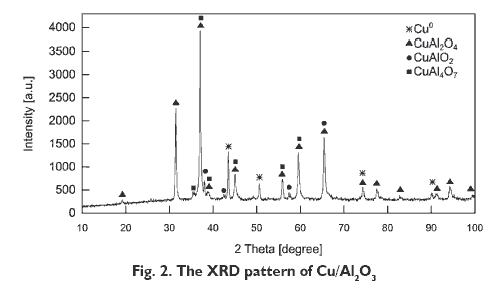
Figure 2 shows the XRD patterns of Cu/Al2O3 catalyst prepared by co-precipitation. The peaks at 2q 43.4°, 50.7° correspond to metallic copper Cu0 and the peaks at 2q 31.3°, 36.9°, 45.0°, 59.4° 65.4° indicate the presence of spinel structure, CuAl2O4. The peaks at 2q 36.9°, 45.0°, 59.4° are also characteristic for CuAl4O7 phase. The peak at 2q 65.4° can be assigned to copper(I) aluminate(III) CuAlO2. The copper crystallite size calculated using the Scherrer equation was 11 nm.
TEM image of the Cu/Al2O3 catalyst (Photo 1.) shows its finecrystalline structure. The structure of catalyst consisted of irregular? shaped particles whose size was estimated to be 8?11 nm.

Surface area of Cu/Al2O3 catalyst determined by BET method was 81.96 m2/g, the average pore volume 0.380 m2/g and the pore diameter 5.12 nm.
Results and discussion
The glycerol conversions and selectivities of transformation to 1,2- and 1,3-propanediol obtained in hydrogenolyses performed in 1- and 2-propanol with in situ generated hydrogen and in the presence of molecular hydrogen are shown in Figure 4.
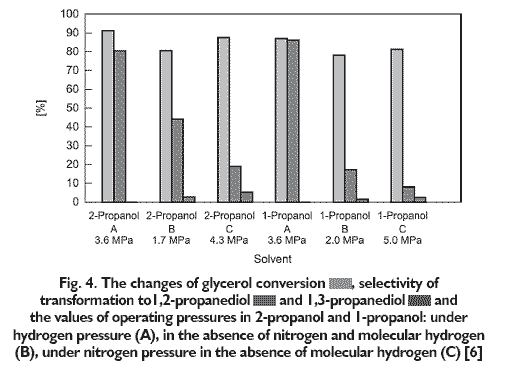
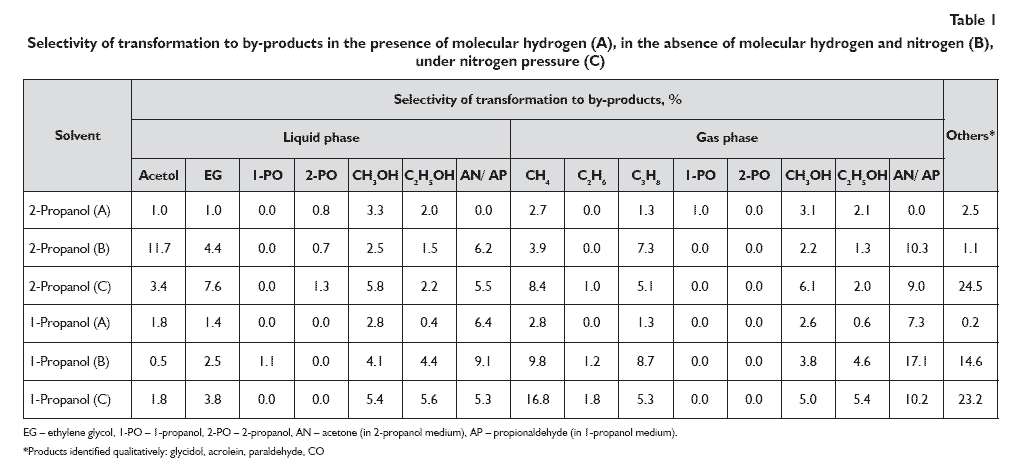
The highest glycerol conversions: 86.9% in 1-propanol, 91.2% in 2-propanol and their relevant selectivities of transformation to 1,2-propanediol: 86.1% in 1-propanol, 80.4% in 2-propanol were achieved in hydrogenolyses carried out in the presence of molecular hydrogen (A). However in both hydrogenolyses 1,3-propanediol was not found in the post-reaction mixture. Unlike to the process performed in the presence of molecular hydrogen, in the pressureless hydrogenolysis, in the absence of molecular hydrogen, both in the 1-propanol and 2-propanol significantly lower selectivities of transformation to 1,2-propanediol and the lowest glycerol conversions: 78.0% and 80.5% respectively were obtained. In the absence of molecular hydrogen and nitrogen (B), in 1-propanol selectivity of transformation to 1,2-propanediol was 17.1%. Thus, it was five times lower in comparison to selectivity obtained under molecular hydrogen pressure. In the absence of molecular hydrogen and nitrogen (B) in 2-propanol selectivity of transformation to 1,2-propanediol amounted to 44.2% which was about half of those obtained in the presence of molecular hydrogen. In pressureless hydrogenolysis (B), in the case of both solvents 1,3-propanediol was obtained, but with low selectivity ? 2.4% in 1-propanol and 2.7% in 2-propanol. The least preferred method of hydrogenolysis was without molecular hydrogen under nitrogen pressure (C). In this method the high glycerol conversions: 81.2% in 1-propanol, 87.4% in 2-propanol were achieved, but at the lowest selectivities to 1,2-propanediol: 8.0% in 1-propanol, 18.9% in 2-propanol. In this hydrogenolysis the highest selectivities of transformation to 1,3-propanediol were obtained, nevertheless they were very low ? 2.4% in 1-propanol, 5.3% in 2-propanol. Moreover, in the process performed under nitrogen pressure, without using molecular hydrogen, the highest increase in operating pressure was observed: 4.3 MPa in 2-propanol and 5.0 MPa in 1-propanol. It was due to the pressure of hydrogen which was formed as a result of dehydrogenation of 1- or 2-propanol [7] and also due to nitrogen pressure which increased after heating reaction mixture to appropriate temperature. The composition of the products obtained after hydrogenolysis (Tab. 1) also shows that the increase in operating pressure was caused also by by-products. Selectivity of transformation to by-products achieved in hydrogenolysis in the presence of 2-propanol and molecular hydrogen (A) is significantly lower, especially with regard to acetol, than in the processes carried out without molecular hydrogen (A or C). This proves that in the presence of molecular hydrogen the hydrogenation of acetol to 1,2-propanediol occurred faster than in the other two methods of performing hydrogenolysis. The different trend is observed for hydrogenolyses in 1-propanol. In the presence of 1-propanol the lowest selectivity of transformation to acetol ? 0.5% was achieved in the pressureless method (B), while in hydrogenolysis performed under hydrogen pressure (A) as well as in the one without molecular hydrogen but under nitrogen pressure (C) the same selectivities of transformation to acetol were obtained. However the pressureless methods gave significantly higher selectivities of transformation to by-products formed from C?C bonds cleavage in glycerol and 1,2-propanediol: ethylene glycol, methanol and ethanol and higher selectivities of transformation to by-products formed in hydrogenation of alcohols, i.e. methane, ethane, propane. Therefore, it can be considered that lower selectivities of transformation to 1,2-propanediol achieved in hydrogenolyses in 1-propanol in the absence of molecular hydrogen (C) resulted from predomination of degradation reactions of glycerol and 1,2-propanediol. Moreover, it is also possible that in the hydrogenolyses performing in the absence of molecular hydrogen, by-products formed as a result of 2- and 1-propanol dehydrogenation, i.e. acetone and propionaldehyde compete with glycerol for the active sites of catalyst [7]. In none of the hydrogenolyses was the 3-hydroxypropanal detected in post-reaction mixture. However by analogy for 1,2-propanediol formation, the mechanism of 1,3-propanediol formation may be proposed (Fig. 1.) also in the case of hydrogenolysis with in situ hydrogen. It may also be assumed that in the presence of the inert gas the activation of ?OH groups on secondary carbon in glycerol proceeds easier, which facilitates C-OH bond cleavage.
Conclusions
Studies of hydrogenolyses using in situ and molecular hydrogen revealed that application of molecular hydrogen is the most advantageous for glycerol hydrogenolysis. Production of 1,2-propanediol by hydrogenolysis of glycerol is clearly more attractive compared to currently used methods, based on petrochemical raw materials. The use of renewable raw material, which is purified glycerol derived from biodiesel production makes the hydrogenolysis process economical and environmentally friendly. Hydrogenolysis performed using molecular hydrogen (A) enables to obtain higher glycerol conversion and significantly higher selectivity of transformation to 1,2-propanediol in comparison to hydrogenolyses carried out using in situ generated hydrogen (B or C). In the processes, when molecular hydrogen under pressure was used, significantly higher selectivities of transformation to volatile by-products were achieved, which proves that in the presence of molecular hydrogen C?C bonds cleavage in glycerol and propanediols is limited. In hydrogenolyses performer in the absence of molecular hydrogen (B and C) 1,3-propanediol was formed, however selectivity of transformation to this products was relatively low. The highest selectivity to 1,3-propanediol: 2.4% in 1-propanol, 2.7% in 2-propaneol was obtained in the hydrogenolysis carried out under nitrogen pressure, without molecular hydrogen.
Literature
1. Cichy M., Borowiecki T., Otrzymywanie wodoru z glicerolu, Przemysł Chemiczny 2009, 88, 9, 995.
2. Gandarias I., Arias P. L., Fernández S. G., Requies J., El Doukkali M., Güemez M. B., Hydrogenolysis through catalytic transfer hydrogenation: Glycerol conversion to 1,2-propanediol, Catalysis Today 2012, 195, 1, 22.
3. Miyazawa T., Kusunoki Y., Kunimori K., Tomishige K., Glycerol conversion in the aqueous solution under hydrogen over Ru/C + anion-exchange resin and its reaction mechanism, Journal of Catalysis 2006, 240, 2, 213.
4. Meher L. Ch., Gopinath R., Naik S. N., Dalai A. K., Catalytic Hydrogenolysis of Glycerol to Propylene Glycol over Mixed Oxides Derrived from a Hydrotalcite-Type Precursor, Industrial & Engineering Chemistry Research 2009, 48, 4, 1840,
5. Mane R. B., Hengne A. M., Ghalwadkar, A. A., Vijayanand, Mohite P. H., Potdar H. S., Rode Ch. V., Cu:Al Nano Catalyst for Selective Hydrogenolysis of Glycerol to 1,2-Propanediol, Catalysis Letters 2010, 135, 1?2, 141.
6. Wołosiak-Hnat A., Milchert E., Nowe Trendy w Naukach Inżynieryjnych 2, pod red. dr inż. Marcina Kuczery, CREATIVETIME, Kraków 2012, 35.
7. Gandarias I., Arias P. L., El Doukkali M., Güemez M. B., Liquid-phase glycerol hydrogenolysis to 1,2-propanediol under nitrogen pressure using 2-propanol as hydrogen source, Journal of Catalysis 2011, 282, 1, 237.
Agnieszka WOŁOSIAK-HNAT ? M.Sc. graduated from the Faculty of Chemical Engineering of the Szczecin University of Technology (2008). Currently she is a PhD student at the Insitute of Organic Chemical Technology of West Pomeranian University of Technology in Szczecin. She is co-author of 7 scientific papers, 4 patent applications and 18 poster presentations at Polish and international conferences.
Eugeniusz MILCHERT ? Professor (Sc. D., Eng),, graduated from the Faculty of Chemical Technology at the Technical University of Szczecin in 1969. In years , he was the Dean of this Faculty. Currently, he is the director of the Institute of Organic Chemical Technology of the Faculty of Chemical Engineering at the West Pomeranian University of Technology in Szczecin. Specialisation ? technology of the basis organic synthesis.
e-mail: ; phone: