Jerzy WASILEWSKI, Jolanta ZIMOCH ? Institute of Heavy Organic Synthesis ?Blachownia?, Kędzierzyn-Koźle, Poland
Please cite as: CHEMIK 2013, 67, 6, 528-539
The problems connected with CO2 emissions as well as the costs of CO2 abatement and the se as a feed for synthesis processes were presented. Modernisation of commercial processes, production of bio-alcohols through fermentation of cellulose, and photosynthesis with the use of algae to produce bio-ethanol, algal oil and bio-diesel, these are promising options for the future energy carriers manufacturing methods.
Introduction
The global economic development, increasing population, increasing demand for electric appliances and consumer electronics, general mechanisation of services and manufacturing processes as well as expanding use of transport facilities, all that contributes to the growing energy demand. Additionally, the resources of conventional fossil energy sources which are limited, rising extraction costs and rising prices of those fuels, and the environmental pollution which is more and more severe, give rise to the general concern over the problem of producing energy in cheaper ways. The factors as listed above attracted the considerable interest over the current two decades in other energy sources and in particular in the renewable energy sources. The importance of that problem is confirmed by the fact that the renewable energy sources are anticipated to take up 20% of the total energy consumption in highly developed EU countries by 2020. Poland also declares that the share of renewable energy sources will increase to 15% in its fuel-energy balance [1]. The policies of EU countries and other developed countries around the world contain the element of expanding the use of renewable energy sources. The importance of the search for new energy sources is also highlighted by the global policy which tends to reduce the CO2 emissions. The rational utilisation of the potential energy resources offered by rivers, wind, solar radiation and biomass, as well as efficient commercial use of geothermal energy, nuclear energy and fuel cells, became the focus of attention for numerous countries. Their governments appropriate considerable funds to support the research and development programmes oriented to the use of renewable energy sources [1÷3]. The present level of energy recovery from natural sources is much different in individual countries and locations. The possibilities in that field are dependent on the insolation conditions, mountainous character of a region, wind power, etc. The costs required for the production of that type of energy make the critical impediment in further development of natural energy generation. That is illustrated in Figure 1 [3].
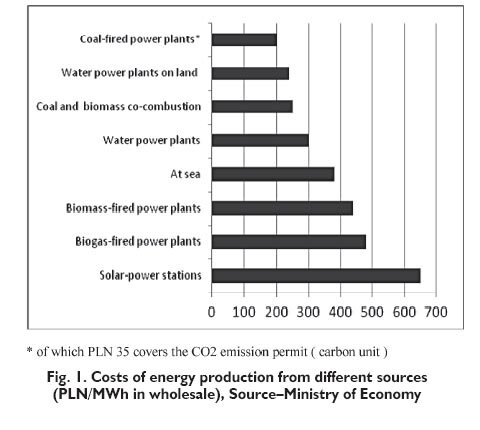
Despite the fact that the technology of alternative energy sources is growing rapidly, coal remains the cheapest energy carrier. Its use, however, is connected with CO2 emission which makes it necessary to improve the combustion technology and even to use sequestration which is expensive. That problem is even more critical since CO2 is claimed to be responsible for the greenhouse effect (that responsibility seems to have been assigned too hurriedly). That opinion has been assumed and presented by the international climate conservation groups. A strong informative and economic pressure from UN climatic commissions and from corresponding national commissions is a driving force for efforts to be made to reduce emissions of carbon dioxide, both in highly developed and in developing countries. That postulate is equivalent to the quest for new methods to produce energy with lower CO2 emissions or with the emissions eliminated completely. The problem is so important as CO2 is considered to contribute to the greenhouse effect, i.e. to add to global warming [4÷8]. According to the Kyoto Protocol (1997), 30 different gases were identified to bring on the greenhouse effect. The Intergovernmental Panel on Climate Change (IPCC), however, recognised with no reliable evidence that carbon dioxide was the principal contributor to the greenhouse effect. The effect of water (vapour) was ignored although that vapour makes a dominant atmospheric component, as illustrated in Table 1 [4].
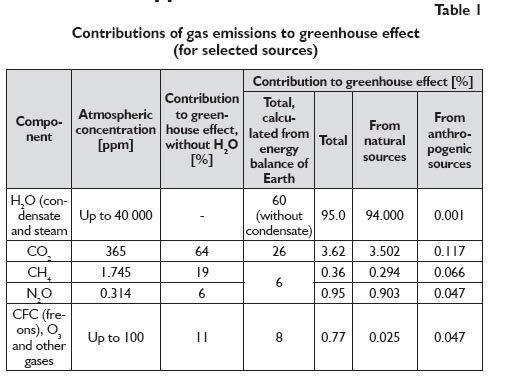
That viewpoint raises objections in the climatological circles, and the group of experts who call that opinion in question grows constantly. The observable global climate changes are attributed to the changes in solar activity by a larger and larger number of opinions [4, 8]. Despite no convincing evidence on the effect of CO2 on the climate change and global temperature increase, some alarming and catastrophic visions are being presented which are based on the reports by the Intergovernmental Panel on Climate Change (IPCC) [7, 8]. They forecast quick melting of glaciers and ice sheets, sea level rise, steppization in various locations on various continents, and higher frequency of extreme weather events (storms, rainfall and snowfall, hurricanes) which cause serious property losses in the affected areas. The effect of CO2 on global warming is not confirmed by the information provided by the atmospheric CO2 concentration measurements and temperature profile records. Figure 2 shows no match for the systematic increase in the atmospheric CO2 concentration over the last decade and global temperature; the latter remains unchanged or it has the tendency to decline [8].

CO2 ? threats and opportunities
The problem of limiting CO2 emissions is hard to handle since the principal CO2 emitting countries refused to sign the obligations to reduce CO2 emissions. Special initiative in making efforts to reduce CO2 emissions is shown by West-European countries which in fact are not responsible for the highest share in CO2 emissions (16 % of global emissions) [9, 11]. Despite reservations about attributing the decisive effect on the global climate changes to CO2, and despite tendentious information published by IPCC on the contribution of CO2 to greenhouse effect, the world?s industry perceives advisability to take measures to reduce CO2 emissions in spite of the need to incur high costs. Poland also spares no efforts to reduce emissions of atmospheric pollutants. Although the capital input to the industrial retrofitting programmes has been considerable, further efforts are necessary to reduce the emissions of greenhouse gases. The levels of CO2 emissions in some countries throughout the world and in European Union, and the costs needed to reduce those emissions in 2013÷2020, have been presented in Figure 3 [8].
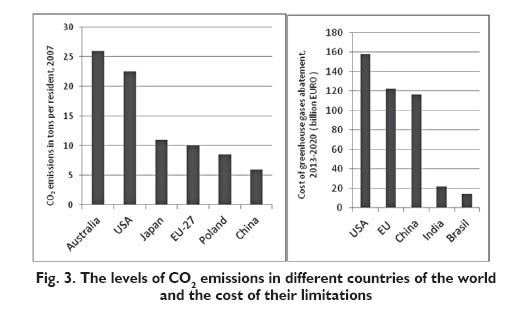
It is especially hard for Poland to attain the established objectives since the input of 92 billion Euros will be required to implement the assumed tasks by 2030. Such high burdens for Poland and for other industrialised countries induces reluctance to carry on what was agreed in the Kyoto Protocol, both in the signatory countries and in those countries which refused to sign. The prospect of such high costs to be borne stimulates some countries and global concerns to search for efficient methods which would reduce CO2 emissions and save energy and raw materials at the same time. That is true in particular for oil companies and chemical companies, as well as for USA, Japan, China and Germany. Those efforts are oriented to direct and indirect energy saving methods, and to the construction and operation of energy efficient equipment and machines, to reduction of emissions of greenhouse gases, and in particular reduction of atmospheric CO2 emissions, as well as to the utilisation of CO2 as a chemical raw material. According to literature data, the principal trends in limiting CO2 emissions cover its physical and chemical utilisation by its storage in underground caverns (sequestration) for further use as a chemical feedstock [10, 11]. The underground storage method is used in USA (Texas) and in Norway. The highest CO2 storage volume in Europe is available in UK: under the Scottish sector of the North Sea. The expert estimates say about 80 to 150 trillion tons CO2. Even bigger CO2 storage volumes are available in Norway. The full usage of that potential storage capacity may fully meet the demand in that field for all European countries over 100 years. However, that method of storage is expensive and it may be employed under favourable geological conditions only [12]. The interest is also growing in the use of CO2 as a solvent and extraction agent. CO2 may be used as a supercritical solvent in the production of fluorinated polymers, as a foaming agent in the production of polyurethane foams, latex, etc. It is also employed in the supercritical extraction process to recover and to concentrate components which are present in various biomass types, as a temperature lowering agent, to generate the inert atmosphere conditions, in deodorisation of animal oils and fats. Moreover, it is used as a shielding gas in welding, and as a solvent in the dry cleaning process for leather, furs and synthetic materials which are sensitive to conventional cleaning agents [10]. The use of CO2 as a raw material is extensively and successfully researched in the developed countries. Germany assigned 100 million Euros recently to support the project ?Technology of climate conservation and protection ? chemical processes which use CO2 as a raw material?. Some important applications for CO2 as a raw material were presented in Figure 4 [11].

The chemical industry saves energy.
The world?s chemical industry takes much care to save energy and raw materials. Costly research programmes have been conducted to improve the existing manufacturing processes which resulted in serious energy savings and reduced volumes of vent gases and waste products. The most outstanding results in that field were achieved through developing new catalysts which were more active and selective, and through the use of cheaper alternative raw materials and/or renewable resources. Those achievements are in particular noticeable in the production of commodity chemicals, and some selected examples were presented in Figure 5 [13]. The implemented innovative technology yielded environmental and economic profits as well as savings in the consumption of energy and raw materials. Similar effects can be obtained by building plants with enormous unprecedented capacities which amount to a few hundred thousand or even a million tons of product per year, and by locating them close to natural deposits of raw materials and close to manpower resources.

The effects of the activities which had been taken by the chemical industry to save energy and to reduce CO2 emissions were evaluated by the International Council of Chemical Associations (ICCA) [14]. That assessment was based on the analysis of 100 selected chemical products over their whole life cycle. The analysis demonstrated that the world?s chemical industry considerably reduced energy consumption and emissions of greenhouse gases. Thus, the volume of chemical products increased by 60% in EU countries in 1990÷2005, with lower energy consumption and with the emissions of greenhouse gases reduced by about 30%. The Japanese optimisation programmes reduced the energy consumption which in 2006 reached the level of 82% of that noted in 1990. In the US chemical industry, energy consumption per production unit dropped down from 1974 by nearly one half, and the emissions of greenhouse gases were reduced from 1990 by 16%. In Brazil, the chemical industry consumed by 25% less energy over 2000÷2007 with the simultaneous growth in the production by about 30%. The chemical industry also provides the indirect possibility of saving energy and reducing CO2 emissions; it produces inter alia the construction insulation materials, washing powders which are effective at lower temperatures, motor fuel additives, etc.
Bioalcohols as a source and a component of fuels
Considerable prospects for saving and obtaining energy emerge from the new methods for the production of energy carriers, i.e. biomethanol, ethanol and butanol. Much interest of chemical companies is attracted by the well-known synthesis method of methanol from syn-gas which can be derived from traditional raw materials and/or from biomass. That interest is reasonable in the light of commercialisation of the Mega-Methanol process which offers a substantial reduction in the methanol manufacturing costs. The name of that technology is representative for the scale of production which is just enormous, i.e. 1,000 or even 5,000 t/d. That high capacity gives also room for the use of considerable amounts of CO2 as the additional feed stream. That process is operated in Iran, in Trinidad and in Qatar, i.e. in those countries where natural gas is cheap. That cost-efficient production of methanol creates the feasible prospects for launching the production complexes in the future for the manufacture of propylene and other olefins together with their derivatives, motor fuels and components of motor fuels, acetic acid, formaldehyde, etc [13].
Ethanol and butanol become more and more important in the raw materials management. They offer savings in obtaining energy, especially after improvements and innovations in the fermentation processes. In particular, the bio-ethanol process is so advanced that ethanol can be produced in practice from any biomass which contains carbohydrates or polycarbohydrates. The vast and diverse resources of raw materials and the production economics attract more and more interest in bio-ethanol as a fuel and motor fuel additive, and as a feed for the production of bio-ethylene and bio-polyethylene. The cost-effective use of ethanol in motor fuels is generally accepted in Brazil, in USA, and also in Europe and in China. Low bio-ethanol prices made it possible to build ethylene plants in Brazil (operated by Braskem, Crystalev and Dow Chemical, and Solvay) with the capacities of 200,000; 350,000 and 60,000 Mg/year, respectively [15÷18]. The ethanol dehydration method to ethylene has not been commercialised in Europe yet because of its manufacturing costs. The position of bio-ethanol as a renewable feedstock for the production of hydrocarbon fuel which is not different in practice from an oil-derived fuel makes it possible to use ethanol in the production of motor fuels according to the process developed by Mobil. That process, known as an ETG (Ethanol-To-Gasoline) process, makes it possible to produce gasoline which meets the European standard for euro-super E-95. It is an important question for the production of bio-ethanol as a second-generation component to develop and commercialise a fermentation-based method for the production of bio-ethanol from cellulose. The latest version of that process was presented by DSM and Poet (USA). The 20 million gallon per year plant is planned to be commissioned in the second half of 2013. Then, that production is planned to be ?copied? in 27 plants operated by Poet, and it may be licenced to other interested companies. It is anticipated that 350÷400 new bio-refineries would be built in the USA by 2022 which would produce 16 billion gallons of ethanol per year. A similar technology is also available to Du Pont.
Extensive research has also been conducted on the fermentationbased method for the production of bio-butanol. Du Pont and BP developed their own processes in which biomass can be converted into bio-butanol. However, the costs are too high for those processes, so those methods cannot be implemented throughout the industry. Despite that, Cobalt Biofuels started its pilot plant in 2009, and Green Biologics and Laxmi Organic Industries planned to build their commercial plants in India. The growing interest in bio-butanol results from its superior usability over bio-ethanol in the fuel industry. The production of bio-butanol is of great importance since it is regarded as a valuable new generation fuel. Moreover, its functional properties are more advantageous than those of bio-ethanol. First of all, its water solubility is lower, its miscibility with gasoline is better, it is less corrosive to metals, and its vapour pressure is lower than that of ethanol. Unlike ethanol, it may be transferred through pipelines and it makes an additive to gasoline and to rape-seed oil [19÷23].
Photosynthesis as an opportunity for bio-fuels
Also, extensive research is conducted on the use of carbon dioxide in the photosynthesis reaction. Those reactions are studied in the laboratories operated by world?s companies, and in Polish laboratories [24, 25]. The photosynthesis process which occurs over a catalyst, e.g. TiO2, and under UV irradiation conditions converts CO2 to methanol and water. Despite positive technical results, that process is not economically viable and cannot be commercialised for the time being. As it was found from the energy balance, 3 kg CO2 would be released to produce energy which is required to utilise 1 kg CO2 in that method [26]. Despite that, the photosynthesis reactions are still studied and US scientists successfully created an artificial leaf which can generate energy via photosynthesis [27]. That device is 10 times more efficient than other naturally occurring processes. One artificial leaf, with the size of a playing card, equipped with the sophisticated electronic systems and reaction catalysts, when placed in water and exposed to direct sunlight, can generate the amount of energy which is needed to supply a house all day long. Its principle of operation is based on decomposition of water to produce oxygen and hydrogen which are then utilised in fuel cells [2]. A prototype device was operated without a break over 45 hours and its efficiency did not decline over that time. That makes a very interesting and possibly prospective method for the production of energy.
Special interest is attracted by algae as a source of fuel and energy [25, 28÷30]. The possibility of using algae has been well known for a long time: they can be used to produce extracts which are then employed in creams, tonics, shampoos and other cosmetics. Algae were found recently to be capable of assimilating considerable amounts of CO2. Being autotrophic organisms, they utilise solar radiation and CO2 to synthesise numerous chemical compounds at ambient temperature. They produce those compounds much more effectively than the cultivated plants can do. Many products, inter alia poly-unsaturated fatty acids, bio-ethanol, bio-butanol, chlorophyll, 1,3-gluten, bio-hydrogen, etc., can be obtained from algae. Algae assimilate considerable amounts of CO2: they use 180 Mg CO2 to produce 100 Mg biomass[30]. Those properties have made algae the focus of growing attention of the world?s chemical and power engineering companies since a few years [31]. The examples of such companies comprise: Algenol Biofuels, Exxon Mobil, Shell, Vattenfall, Bio Fuel Systems (BFS), power concern ENI, and Europeische Algen Biomasse Verband in which about 40 companies and academic institutes have been associated [31, 32].
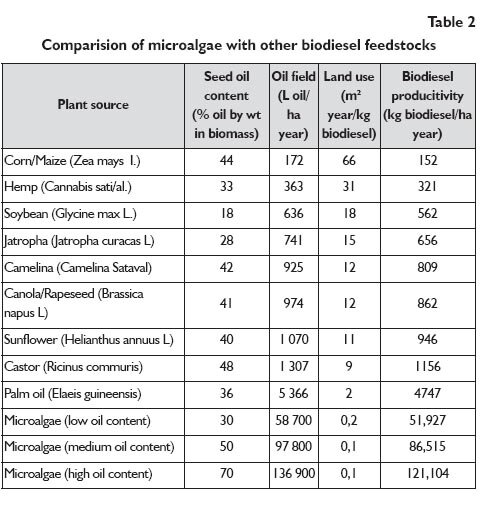
Special interest in obtaining bio-fuels from algae was demonstrated by Exxon Mobil; they assigned $600 million to develop that process. Research in that field can potentially be successful ? over 100 companies show interest in algae throughout the world. It is an important factor which adds to the idea of utilising algae: the third generation biofuel was obtained from them. Such a bio-fuel can be produced from microorganisms which do not compete with the products which are utilised in the production of foodstuffs [33÷35]. Algae were moreover found to produce biomass via photosynthesis 6÷12 times faster than oil plants (corn, soya, rape, special grass types). The advantage of algae over oil plants is illustrated in Table 2. That applies in particular to the oil yield per hectare [32]. The set of identified algae numbers about 30,000 species, of which a few hundred may be of industrial importance. Algae grow through photosynthesis, in the presence of solar radiation and CO2. Their multiplication process is controlled by temperature, insolation, pH of medium, nutrient medium and type of reactor [29, 36]. The optimum algal growth takes place at 20?24°C, at pH=7.0 to 9.0, in the medium where the nitrogen, iron, phosphorus and silicon compounds are present. Algae may be cultivated in freshwater and in seawater. For those species which grow in seawater, the optimum salt concentration falls within 20?24 g NaCl/l nutrient medium. It is recommended to provide uniform illumination of the medium, optimum agitation and supply of air with the CO2 content up to 1%. The oil recovered from algae may be obtained by breaking the algal cells. But the genetic engineering methods were employed recently so that spontaneous release of oil and its accumulation on the surface of the reaction mixture became available. That process was named biomanufacturing, as distinguished from ordinary forming [34].
The block diagram for the production of bio-diesel from algae is presented in Figure 6 [32]. Algae may be cultivated in open systems (open raceway ponds) or in closed systems (photo-bioreactors) [29, 30].

An Open Raceway Pond (ORP) is built in the form of a closed loop channel, 0.3 m deep (Fig. 7) [39], and provided with the equipment to agitate the medium and to control the process parameters. The extended surface provides good exposure to light. That system has some drawbacks: low concentrations of the biomass can be obtained only, water evaporates from the system and other organisms can grow in it. Those drawbacks can be eliminated or limited by the use of closed systems. Photo-bioreactors (PBs) are used for that purpose [29, 40, 41]. These are composed of light-transparent tubes through which the cultivation medium is passed; it is also subjected to agitation. After recovery of algae from the system, the carrier liquid is recycled to the system and the nutrient medium is supplemented. The yield of biomass is affected predominantly by carrier liquid flow rate. The example of a photo-bioreactor system is presented in Figure 8 [29, 39].


Photo-bioreactors offer a few times higher crop of algae than open systems (ORPs). The features of both those systems were compared in Table 3 [32]. The provided information suggests some drawbacks of both the systems. Despite that, the algal cultivation technology is studied extensively in numerous research centres throughout the world. The principal purpose of the research programmes is to reduce the algal biomass production costs and downstream processing costs. The extensive research on the use of algae and carbon dioxide in the production of bio-fuels is also conducted in Europe [27]. Bio Fuel Systems (BFS) (Spanish-French Company) built 400 eight meters high reactors close to Alicante. They produce artificial oil which is then processed to produce fuels with the properties similar to those of oil-derived fuels (Tab. 4) [30].

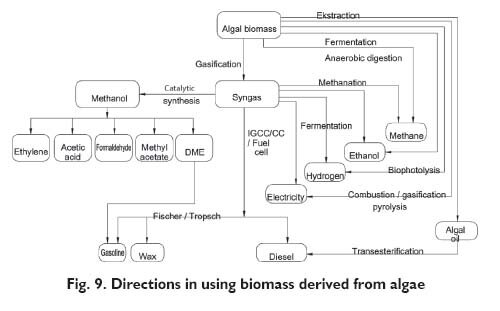
The research findings were utilised in interesting calculations. A company with the area of 50 km2 may produce 1.25 million barrels of ?green? oil per day [27]. That makes a considerable volume in the fuel market of many countries. The information was given out that the price of diesel oil obtained from algae will not be higher than ? 25/barrel, while that figure for gasoline is 40. A spokesman of that company said that the production could be commercialised within 5÷10 years [34, 40]. The algal biomass may be processed with the use of known methods and many valuable products may be obtained. The biomass processing diagram was shown in Figure 9 [41].
Vattenfall (Germany) is developing its process which is similar to that of BFS [27]. Extensive research has also been conducted on the use of bluegreen algae in the production of bio-ethanol from H2O and CO2 through photosynthesis [30, 31]. Dow Chemical appropriated $50 million for the construction of a pilot unit. Together with Algenol Biofuels, they implement the ethanol process which utilises CO2, water and blue green algae [31, 34]. The process is conducted in the similar way as the production of bio-fuels: the reaction is ?driven? by the sunlight and algae; the latter have been genetically modified to make them resistant to higher concentrations of ethanol and salt water. Ethanol is to be recovered in a membrane separation process and not by distillation. Algenol Biofuels also studies the Direct-To-Ethanol process together with the Humboldt University of Berlin, and it is building a 380,000 l/year ethanol plant in Florida. The plant comprises 3,000 bioreactors made of a transparent material, 15 m long and 1.5 m wide each. The ethanol concentration is increased to 99.7% in a membrane separation process, and that product is applicable as a fuel. The company?s management defined that the direct-to-ethanol process would be cost-effective at the price of $100 per oil barrel [31]. Extensive research on new energy carriers which do not compete with the food industry (I generation fuels) is especially important in the light of the latest European Union suggestions to limit the biocomponent level in the fuels down to 5% [42, 43]. That decision would save the foodstuffs to satisfy the food demand from the population of 127 million. On the other hand, however, that decision would close down the plants which produce I generation fuels and would increase the unemployment figures.
Summary
The growing demand for energy and fuels, with the growing prices of energy carriers, drive the more extensive research on renewable energy carriers and drive energy savings. No efforts in that field were also sparred by the chemical industry which makes a considerable energy consumer. The initiatives covered up-grading the existing processes, changes in the investment policies and quest for new energy carriers, inclusive of those which make points of interest for the food industry. In the light of the research findings, the processes seem perspective which convert cellulose to yield bio-ethanol and bio-butanol, and which make use of genetically modified algae to produce bio-ethanol and ?green? oil. Experimental algae cultivation and bio-fuels production plants have been operated in a few countries, and their results augur well for their commercialisation within 5÷10 years. Presented research subject area was also undertaken, although with a delay, by some domestic (research) centres. Furthermore the intellectual and technical potential justifies the success expectations, however in the further future than declared by the western centres.
Literature
1. Tytko R., Odnawialne źródła energii, OWG Warszawa, 2009.
2. Piela P., Czerwiński A., Przegląd technologii ogniw paliwowych, Przem. Chem., 2006, 85/1, 13?18 , 85/3, 164?170.
3. Zasuń R., Czy lasy pójdą na prąd, Gazeta Wyborcza. Gospodarka 2012. 03. 21.
4. Gosiewski K., Antropogeniczne globalne ocieplenie w świetle wiedzy, Przem. Chem. 2009, 88/9, 898?906.
5. Gruszka A., CO2 ? tlenkiem szczęścia czy nieszczęścia, Chemia Przemysława, 2008 nr 6, 17?19.
6. Pietrzak R., Ditlenek węgla a efekt cieplarniany. Manipulacja medialna czy rzeczy-wista groźba. Przem. Chem. 2012, 91/2, 125?127.
7. Teluk T., Mitologia efektu cieplarnianego, Fundacja Instytutu Globalizacji, 2008.
8. Bednarz L. M., Kopenhaga. Polityka i środowisko, Przem. Chem. 2010, 89/3, 187?189.
9. Owczuk M., Biodiesel a ochrona środowiska, Przem. Chem., 2009, 88/3, 240?242.
10. Ogonowski J., Skrzyńska E., Ditlenek węgla, obawy, zagrożenia i nadzieje. Chemik, 2007, 60, 426?429.
11. Wasilewski J., Ditlenek wegla ? zagrożenia i perspektywy, seminarium Platforma Innowacji Technologicznej, Opole 2011. 12. 08. PITRO
12. Anonim, Morze Północne ? potencjalne składowisko CO2, Przem. Chem., 2009, 88/11, 12?13.
13. Wasilewski J., Zimoch J., Ekologiczne aspekty rozwoju technologii chemicznej organicznej, Przem. Chem., 2009, 88/9, 986?995.
14. Anonim, Udział przemysłu chemicznego w dekarbonizacji gospodarki, Przem. Chem., 2010, 89/2,115.
15. Anonim, DSM rozwija produkcję bioetanolu celulozowego. Przem. Chem., 2012, 91/4, 486.
16. Mccoy, Diversa and Celunol plan new player in cellulosic etanol, Chem. Eng. News 2007,85/8, 11; [Przem. Chem., 2007, 86/5, 449].
17. Anonim, Etanol celulozowy ? paliwo przyszłości, Przem Chem., 2012, 91/3, 433.
18. Anonim, Etanol celulozowy w USA. Zamierzenia i rzeczywistość, Przem. Chem., 2009, 88/8, 916?917.
19. Anonim, Butanol jako paliwo i półprodukt chemiczny, Przem. Chem., 2007, 86/7, 814?815.
20. Garncarek Z., Kociołek-Balawajder E., Biobutanol. Perspektywy rozwoju produkcji, Przem. Chem., 2009, 86/6, 658?666.
21. Smerkowska B., Biobutanol ? produkcja i zastosowanie w silnikach Diesla, Chemik 2011, 65/5, 549?556.
22. Capoy A., Du Pont i BP pracują nad nowym biopaliwem, The Wall Street Journal Polska ? dziennik finansowy, 2008. 07. 31.
23. Anonim, Butanol jako paliwo i półprodukt chemiczny., Przem. Chem., 2007, 86/7, 814?815.
24. Nazimek D, Czech B., Sztuczna fotosynteza ? sposób na zagospodarowanie emisji CO2, VII seminarium ?Ochrona Środowiska?, Kraków 2009. 10.12.
25. Krzemińska J., Tys. J., Mikroglony jako źródło biomasy energetycznej, Chemik, 2012, 66, 12, .
26. Pawłowski L., Nie finansujemy perpetum mobile, Gazeta Wyborcza, 2009. 09. 06.
27. Kościelniak P., Ekologiczna ropa naftowa, Rzeczpospolita, Nauka, 2011. 04.05, A18.
28. Chisti Y., Biodiesel from microalgae, Biotechnology Adwances, 2007, 25, 294?306.
29. Cieśliński H., Biomasa z alg, Chemia Przemysłowa 2012, nr 3, 46?51.
30. Igliński B., Buczkowski R., Piechota G., Algi. Źródło substancji chemicznych, Przem. Chem., 2011, 90/6, .
31. Anonim., Biopaliwa III generacji, Przem. Chem., 2012, 91/9, .
32. Mata. T. M., Martins A.A., Caetano N.S., Microalgae for biodiesel production and other applications: A review, Renewable and Sustainable Energy Reviews 2010, 14, 217?232.
33. Voith M., Start ? up companies see algae as the new renewable fuel source, Chem. Eng. News, 2009, 87/4, 22?23; [Przem. Chem.. 2009, 88/5, 623? 624].
34. Voith M., Project will tests a process to tourn CO2 into ethanol, Chem. Eng. News, 2009, 87/27, 10; [Przem. Chem. 2009, 86/9, 979] 35. Anonim, Biodiesel z glonów, 2007, 86/9, 926.
36. http://www.growing-algae.com./algae-growing-conditions.html, Algae Growing Conditions.
37. Grima E. M., Fernandez F. G. A., Camacho F. G., Chisti Y., Photobioreactors: light, regime, mass transfer and scaleup, Journal of Biotechnology, 1999, 70, 231?247.
38. Travieso L., Hall D. D., Rao K. K., BenitezF., Sanchez E., Borja R., International Biodeterioration and Biodegradation, 2001, 47, 151?155.
39. https://newbusiness . grc.nasa. gov/files/2008/12/ben-amotz-nasa-nov-2008, pdf, Bio-fuel and CO2 capture by algae.
40. Kowalski K., Paliwo do aut z niczego, Rzeczpospolita ? Nauka, 2011. 03. 3, A 18.
41. www. Algae.com
42. Kublik A., Unia ma dość biopaliw., Gazeta Wyborcza, 2012. 09. 19.
43. Dziennik radiowy, program I ? wiadomości, 2012. 10. 18 on 6AM.
Jerzy WASILEWSKI ? Professor ( Sc.D, Eng), graduated from the Faculty of Chemistry, Łódź University of Technology, in 1958. He is Plenipotentiary of the Director of the Institute of Heavy Organic Synthesis ?Blachownia? in Kędzierzyn-Koźle. He specialises in organic chemical technology and industrial catalysis.
Jolanta ZIMOCH ? Ph.D., (Eng), graduated from the Faculty of Chemistry, Silesian University of Technology, in 1985. She obtained her doctor?s degree at the Faculty of Chemical Technology, Poznań University of Technology (1999). She is Assistant Professor at the Institute of Heavy Organic Synthesis ?Blachownia? in Kędzierzyn-Koźle. She holds the position of the Manager of the Expertise and Services Department. She specialises in technology of detergents. e-mail: , phone:
