Bartosz LEŚNIAK, Łukasz SŁUPIK, Grzegorz JAKUBINA ? Institute for Chemical Processing of Coal, Zabrze, Poland
Please cite as: CHEMIK 2013, 67, 6, 560-571
The article discusses the coal properties having influence on its specific heat capacity. The literature correlations for the determination the specific heat capacity of coal, depending on the temperature and the volatile matter content, are presented. To examine the correlation, calculations for several coals of known properties were made and obtained results are showed in the form of a specific heat ? temperature diagram. The received results of the analysis of correlations and suggestions for its range of application are presented.
Introduction
The optimization of chemical processing of coal requires a lot of research and analysis. Understanding the phenomena, that occur during the process of coal gasification or pyrolysis, allow to control them in such a way, as to achieve the best results, which is the highest quality product with minimal energy consumption and no emissions into the environment. Increasingly, for this purpose, mathematical models of the process are used. The model of process in the coke oven chamber is being constructed at the Institute for Chemical Processing of Coal and realized within the framework of a key project ?Smart coke plant fulfilling requirements of best available techniques?. The coupled numerical model of thermal-hydraulic processes occurring during the carbonization of coal in the coke oven chamber includes diffusive, convectional and radiative heat transfer, as well as flow of the gas in porous material which the coal is. In addition the evaporation and condensation of moisture are also included as well as the release of volatiles and additional effects, which are derivative of pyrolysis, such as a change of the charge density, transverse shrinkage and formation of the plastic layer and its movements. To be able to simulate the most reliably coupled processes of the heat and mass exchange with chemical reactions occurring at the same time, is necessary to know the changes in various parameters with increasing of temperature. One of the basic parameters necessary to describe the accumulation element of discretized energy equation is the specific heat capacity. Mathematical modeling of coking coal process comes up with a number of difficulties. In the case of complex organic matter such as coal, we have to deal with the structure of unidentified chemical compounds that are also contaminated with a mineral substance. Thus, even the selection of the necessary thermodynamic parameters of the coked coal model represents a number of questions. Theoretically, it is possible to obtain a part of data by laboratory tests. However, due to the limited possibility of obtaining the sufficiently wide spectrum of data in this way, these parameters are usually given in different correlations that capture the series of known characteristics of coal. One of the relevant thermodynamic parameters of coal, which is the component of physical internal energy is the heat capacity. The value of the specific heat capacity of any substance depends mainly on temperature [1]. In the case of coal, its carbon content, the moisture content, volatile matter, and the composition and content of ash have great influence on the specific heat capacity value. Specific heat capacity of coal usually increases with moisture content, decreases with carbon content and increases with volatile matter content [2].
Specific heat capacity of coal as a function of temperature and volatile matter
Heat capacity is the quantity of heat required to change the temperature of substance by a given amount. The international SI unit of heat capacity is J/K. This value is dependent on the amount of the substance. Derivatives values include molar heat capacity, which is the heat capacity per mole of the pure substance and the specific heat capacity (more properly referred to as a mass specific heat capacity and less accurately the specific heat), which is the heat capacity per unit weight. These values are intensive, which means that they are not dependent on the amount, but the type of material, as well as physical conditions of heating. In the case of coal at elevated temperatures irreversible changes of carbonaceous material associated with the release of volatile matter: coal ? solid carbonaceous material+released volatiles. For this reason, determination of the specific heat capacity of coal as a function of temperature is a complex one. Using the following formula for calculating the specific heat capacity of the coal charge in the temperature range of 100?300oC was proposed by Hoffherr [3]

For the determination of the specific heat capacity of coal, approach presented by Kirov was used by Eisermann [4]: coal is separated into the following component substances: fixed carbon, primary volatile matter (released at lower temperatures), secondary volatile matter (released at higher temperatures), ash and moisture. Assumed, that the volatile matter in a dry, ash-free basis exceeding 10% should be considered as the primary, and up to 10% as the secondary volatile matter. For example, if the coal contains 26% volatile matter in the dry, ash-free basis that means that the primary volatile matter is 16%, and 10% is the secondary. If the volatile matter content is less than 10%, there are only the secondary volatiles. The specific heat capacity of fixed carbon in the dry, ash-free basis according to Eisermann proposition can be calculated by the following equation:

In turn, the specific heat capacity of primary and secondary volatile matter can be calculated by the following equations:

The total specific heat of coal in the dry, ash-free basis is the sum of the above components:
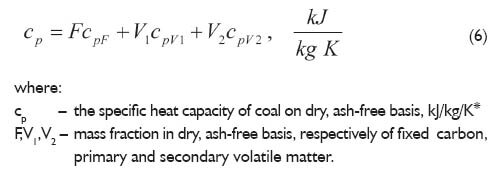
Recently, the correlation for the determination of the dependence of the specific heat capacity of coal most often quoted in the literature [5÷7] is based on Merrick?s model [8]. This model, based on Einstein?s quantum theory of solids, describes the specific heat of coal as a function of temperature T, the molar mass of coal substance M and Einstein characteristic temperature ?:

The reciprocal of average molar mass can be determined by:

To determine the specific heat capacity of coal, Richardson [5] applied differential scanning calorimetry (DSC). Measurements for 26 different coals in the temperature range of 300?600K were made. Based on that data, polynomial correlations describing the specific heat of coal as a function of the temperature and volatile matter were developed:
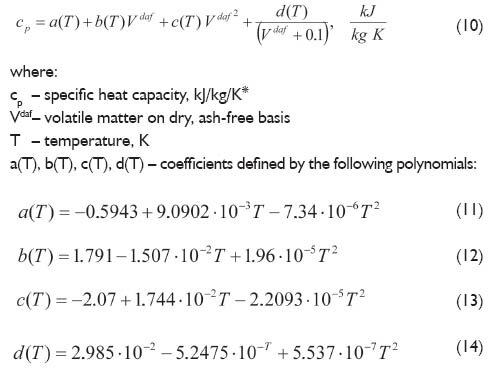
In turn, Postrzednik [9], on the basis of the literature data concerning the measurements and research of the specific heat of coal, developed the following correlation to determinate the specific heat capacity of coal as a function of the temperature and volatile matter content on dry, ash-free basis:

Ballast impact on the specific heat capacity of coal
The main components of coal are combustible, ash and moisture. The resultant specific heat capacity of coal is the weighted average of components mass fraction [5]:

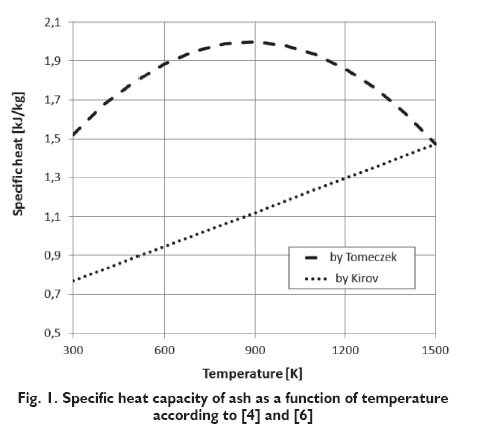
Due to the fact that combustible is the main component of coal, to determine its specific heat exact determination is required. The specific heat of ash and moisture can take approximate values. According to Postrzednik [9], value of the specific heat of ash is respectively 950 J/kg/K*. In the Kirov approach [4], applied also by Merrick [8], the specific heat capacity of ash contained in the coal is determined according to:

However, Tomeczek [6] on the basis of the tested samples, established the following formula for the specific heat capacity of mineral matter (ash):

Values obtained according to the above formulas are shown in Figure 1. In the case of formula proposed by Tomeczek the specific heat capacity of the ash are up to 900 K almost twice as high as in the case according to the formula proposed by Kirov. For formula proposed by Tomeczek, at about 900 K temperature can be observed a maximum. At higher temperatures the value of the specific heat decline and in the a temperature of 1500 K achieve the same value as for the function proposed by Kirov.
Analysis of selected correlations to determine the specific heat capacity of coal
The analysis of correlation for several coals with different properties shown in Table 1 was made.

For each of examined coals, the specific heat capacity on dry, ash-free basis were calculated at the given temperature. Changes in the specific heat capacity of the combustible in the coal on dry, ashfree basis, as a function of temperature in the range of 300 to 600 K are shown in Figures 2÷5. Assuming that there are no changes of carbonaceous material in this range of temperature, the composition of coal does not change. The specific heat capacity depends only on the temperature and basic parameters of coal. In Figures 3÷5 the lines that show the change of the specific heat capacity on the graph for Janina coal coincide with the lines for Bobrek-Centrum coal.


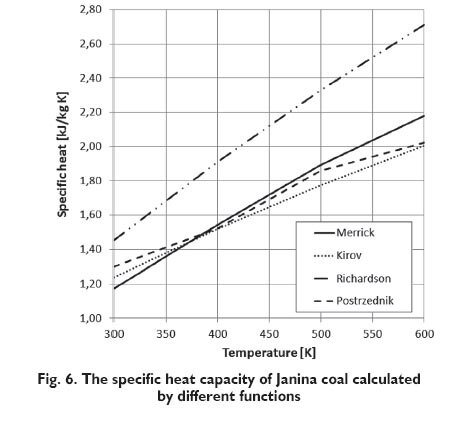
The results obtained for examined coals, as it is apparent from the following diagrams, are different depending on the considered functional describing the change of the specific heat capacity as a function of the selected parameters. However, the specific heat capacity increases with the volatile matter content and decreases with carbon content for the Kirov?s and Postrzednik?s function, which is consistent with the previously presented Speight?s publication [2]. In the case of the function proposed by Richardson, impact of this coal parameters on the specific heat capacity is quite the opposite. Furthermore, the values of specific heat capacity, calculated from Richardson?s function for temperature range to 600 K are much higher than those calculated on the basis of other functions. This difference is showed on the basis of the specific heat capacity change of ?Janina? coal calculated by the different functions (Fig.6) In the case of functions proposed by Kirov and Postrzednik, it can also be noted that the volatile matter has a greater impact on specific heat than the carbon content. While in the case of the function proposed by Merrick, carbon content has greater impact than volatile matter. In the Figures 7÷9 the results of the same functions in the temperature range from 300 to 1300 K are presented. It was assumed that, in this temperature the decomposition of coal material occurs and is connected with the change of the volatile matter content. Because of dissimilarity, Richardson?s function is not included in these charts. For the determination of change taking place in the coal, the simulation calculations using the model of pyrolysis developed by Ściążko [10] were made. This model allows to calculate the change of the efficiency of each evolved volatile matter component for any coal and for any arbitrary heating rate. According to the author?s thesis, the efficiency of a particular component is proportional to the amount of volatile matter, and the closing model equations is the mass balance of elements. Definitively, the generalized model of pyrolysis allows to calculate kinetic parameters of the overall volatile matter and its components and to determine the amount of exuded volatile matter and its component at a given temperature. As a result of simulations, the properties of chars obtained from each coals in the temperature range from 500 to 1300 K were obtained.
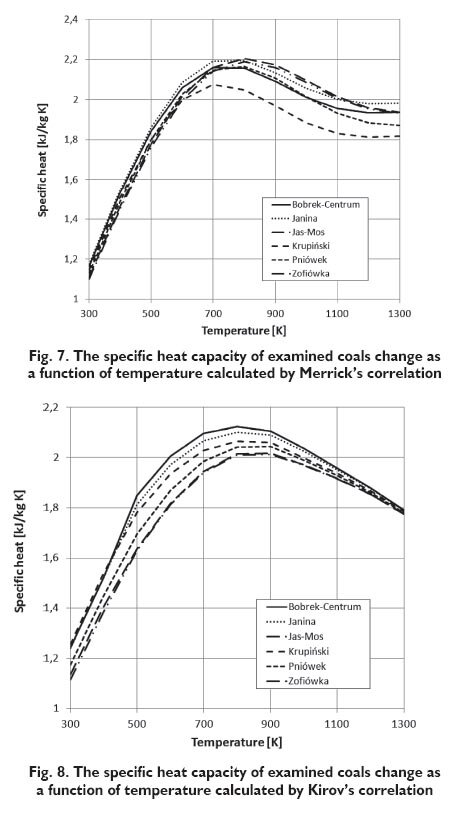
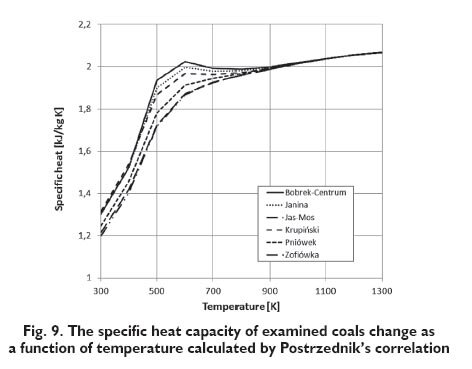
As it is apparent from the presented diagrams, the character of curves illustrating the change of the specific heat capacity as a function of temperature proposed by Merrick and Kirov is similar. The value of specific heat capacity is increased and reaches the maximum of all the examined coals in the temperature range of 700?800 K. Above this temperature, the specific heat capacity of coals begins to decrease. In the case of Postrzednik?s function, the character of curves is different. The specific heat capacity increases across the whole range, however above the temperature range of 600?700 K the increase is insignificant.
The following Figures 10÷12 show the same results for the ?Krupinski? coal but with three various assumptions:
? the decomposition of coal that takes place under the influence of temperature is included (coal composition is changing with temperature)
? the decomposition of coal that takes place under the influence of temperature is not included (coal composition isn?t changing with temperature)
? coke obtained from the coal.
For comparison, the specific heat capacity of graphite as a function of temperature according to two [11,12] of several known correlations is also presented on the diagram:
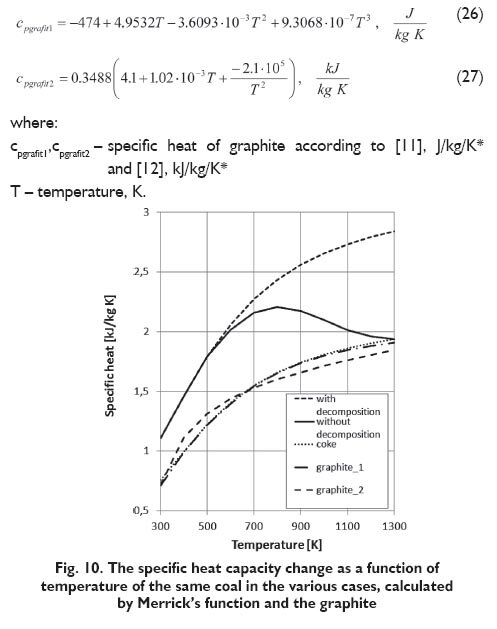

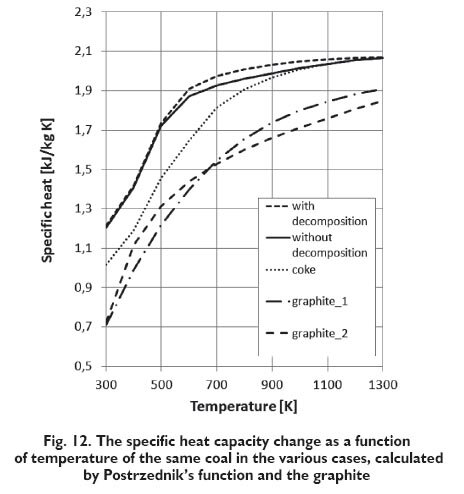

In both case of the functions proposed by Kirov and Merrick considering or not carbonaceous substance changes in elevated temperature, leads to significantly different results. Disregarding of carbonaceous material changes with temperature, and consequently changing its composition during application of these functions, makes that the calculated specific heat capacity increases in the whole investigated temperature range (Figs. 10, 11). There is a different in the case of function proposed by Potrzednik. In that case both considering or not the changes, there is almost no influence on obtained result (Fig. 12). In the case of determining the specific heat capacity of coke, both in case of application the Merrick?s as well as Kirov?s function, obtained values are substantially similar to those obtained for graphite. If the changes of carbonaceous material are taken into account obtained values of the specific heat capacity at temperatures above 500 K are beginning to fall and strive to values obtained for coke (Figs. 10,11). For function proposed by Postrzednik calculated values for coke differ significantly from the values for graphite (Fig. 12). In the Figure 13 for comparison, the results obtained from the three function, both with considering the changes and without its considering are presented. As can be observed the character of curves for function proposed by Kirov and Merrick are similar to each other, but for the Kirov?s function obtained values are of about 10% lower than according to Merrick?s. The values of specific heat capacity determined from the function proposed by Postrzednik are close to the values calculated by using the Kirov and Merrick, taking into account the changes.
Summary
In the article an analysis of four different functions used for determining the specific heat capacity of coal as a function of temperature is presented. For the analysis six coals were used. The simulation calculations were carried out, using the pyrolysis model developed by Ściążko to obtain the composition of chars in the investigated temperature range. For the received chars the calculations of the specific heat capacity using a presented function were made. As is apparent from the analysis, for the functions proposed by Kirov as well as Postrzednik, specific heat capacity of coal increases with content of volatiles and decreases with carbon content, which is consistent with presented Speight?s publication. In the case of function proposed by Richardson, the impact of these parameters on the specific heat capacity is quite the opposite. Furthermore the values of the specific heat capacity, calculated from the function proposed by Richardson at the temperature range from 300 to 600 K are much higher than those prescribed on the basis of the other functions. Due to the limitation of the temperature range of applicability, the function proposed by Richardson was not analyzed at temperatures higher than 600 K. As was shown by further analysis, the character of change the specific heat capacity, calculated using functions proposed by the Kirov and Merrick is similar to each other. In the case of using these functions significant influence on the obtained values of the specific heat capacity is fact of considering carbonaceous substance changes with temperature. In the case of Postrzednik?s function this influence is negligible.
Translation into English by the Author
Literature
1. Szargut J.: Termodynamika techniczna. Wydawnictwo Politechniki Śląskiej, Gliwice, 2010.
2. Speight J.G.: Handbook of Coal Analysis. Published by John Wiley&Sons, New Jersey, 2005.
3. Hoffherr K., Simonis W.: Der Zusammenhang zwischen der zeitlichen Anderung der Temperaturfelder bei der Hochtemperaturaturverkokung von Steinkohle im Laboratorium, im Technikum und im Betrieb. Glückauf Forschungshefte 1971, 32.
4. Eisermann W., Johanson P., Conger W.L.: Estimating thermodynamic properties of coal, char, tar and ash. Fuel Processing Technology 1980, 3, 39?53.
5. Coimbra C.F.M., Queiroz M.: Evaluation of a Dimensionless Group Number to Determine Second-Einstein Temperatures in a Heat Capacity Model for All Coal Ranks. Combustion and Flame 1995, 101, 209?220.
6. Tomeczek J., Palugniok H.: Specific heat capacity and entalphy of coal pyrolysis at elevated temperatures. Fuel Vol. 75, No. 9, 1996.
7. Tang H., Guo Z., Guo X.: Numerical model of coal carbonization analysis of a coke oven charge using PHOENICS. Proceedings of PHOENICS 10th International User Conference, Melbourne, Australia, 2004.
8. Merrick D.: Mathematical models of the thermal decomposition of coal 2. Fuel Vol 62, May 1983.
9. Postrzednik S.: Analiza termodynamiczna procesu odgazowania paliw stałych. Zeszyty naukowe nr 691, Politechnika Śląska, Gliwice, 1981.
10. Ściążko M.: Modele klasyfikacji węgla w ujęciu termodynamicznym i kinetycznym. Rozprawy i monografie, 210, Wydawnictwa AGH, Kraków, 2010.
11. http://aries.ucsd.edu/LIB/PROPS/PANOS/c.html, grudzień 2012.
12. Szarawara J.: Termodynamika chemiczna. WNT, Warszawa, 1969. Work realized within a framework of key project No. POIG.01.01.02-24- 017/08 ?Smart coke plant fulfilling requirements of best available techniques? financed by European Regional Development Fund ? ERDF
Bartosz LEŚNIAK ? M.Sc., is a graduate of the Faculty of Power and Environmental Engineering, Silesian University of Technology (2010). He has been working for the Institute for Chemical Processing of Coal in Zabrze since graduation. Competence area includes a system of coke oven battery monitoring and operation control as well as development of simulation models. He is co-authored of three articles in the technical ? scientific press. e-mail: ; phone:
Łukasz SŁUPIK received M.Sc degree in Power Engineering from the Silesian University of Technology. He is currently working at the Institute for Chemical Processing of Coal in Zabrze. His research interests include mathematical modelling of thermal and flow processes in coking chambers. e-mail: ; phone: +
Grzegorz JAKUBINA ? M.Sc., is a graduate of the Silesian University of the Faculty of Power and Environmental Engineering, specialization Environmental Engineering. In 2009, he completed postgraduate studies at the University of Science and Technology in Cracow at the Department of Fuels and Energy on ?Modern Methods of Management and Technology in coke-making.? He has been working for to the Institute for Chemical Processing of Coal as a senior engineering technical specialist and project manager for seven years. He co-authored 5 articles and a monograph. He is also the author or co-author of 5 papers and 3 posters at national and international conferences. Competence areas include :construction, operation and modernization of coke oven batteries. e-mail: ; phone: +
