Janusz LASEK, Aleksander SOBOLEWSKI ? Institute for Chemical Processing of Coal (IChPW), Zabrze
Please cite as: CHEMIK 2013, 67, 6, 514-527
The article presents the state of art on thermal bio-refinery in context of pilot and industrial scale deployments. It presents classification and basic characteristics of the unit processes in thermal bio-refinery, techno-economic analysis based on available data and assessment of development of different types of bio-refinery.
Introduction
According to the definition proposed by the US National Renewable Energy Laboratory (NREL) ?bio-refinery is an integrated system of processes and equipment used to produce fuels, power and chemicals from biomass? [1]. Bio-refinery is therefore a system in which multidirectional and complex conversion of biomass is made. Implementation of thermochemical processes, such as gasification or pyrolysis, is one of the ways of converting biomass to obtain valuable products in bio-refinery. Application of thermo-chemical processes can take place within a single, comprehensive concept [2], or be part of a larger system, called ?two platforms?, which is divided into two paths: the bio-chemical and thermo-chemical conversion processes [3, 4]. Interest of researchers in bio-refinery topic in recent years has gained a particular importance, as it is shown by the number of publications in this field of research; still this issue is not widely discussed in the Polish scientific literature. Usually, R&D works in the field of bio-refineries are mainly focused on the ?purely? chemical aspects (in terms of feedstock-products). In order to meet the expectations of Polish readers, we decided to present given review, taking into consideration the latest scientific literature sources of international scope. Due to the very broad range of the bio-refinery aspects, it was assumed as a principle that they will be described as a system, taking into consideration only basic information concerning the particular stages of the whole process. The following order of the article was assumed: presentation of division on different types of bio-refineries, a simplified description of selected types of bio-refineries and a detailed description of the thermal bio-refineries. The reader, who is interested in more detailed information we invite to study the cited literature, especially the works of Kamm [5, 6], Naik [4], Fernando [7], Aresta [8] and Menon&Rao [3].
2. Division of the bio-refineries
Division of bio-refineries can be made according to several criteria. So far, in the literature, there is no clear division of the bio-refineries or their systematic. Ree and Annevelink [9] presented the following division of bio-refineries:
? Conventional Bio-refineries (CBR)
? Green Bio-refineries (GBR)
? Whole Crop Bio-refineries (WCBR)
? Ligno Cellulosic Feedstock Bio-refineries (LCFBR)
? Two Platform Concept Bio-refineries (TPCBR)
? Thermo Chemical Bio-refineries (TCBR)
? Marine Bio-refineries (MBR).
Naik et al. [4] propose a different division, due to the progress of technology (estimated by the so-called ?flexibility of the process?), used substrates and obtained products. This division introduces gradation between different types of bio-refineries. Within the first categorization (process flexibility) one may single out biorefineries of I, II and III generation. It should be noted that the above mentioned division is not the same as the distribution of biofuels for generations. Bio-refineries of the first generation include systems in which substrates are used in dry batch form of a similar type of a specified graining (e.g. cereal grains), and as the final product we may obtain ethanol, post fermentation substance (called Distillers Dried Grain, DDG) and CO2. This system is characterized by the minimal process flexibility. Bio-refineries of the second generation use the same feedstock, but processes are more flexible and productive. The final products may be starch, glucose-fructose syrup, ethanol, post-fermentation substance, CO2 and oil. Bio-refinery of the third generation are systems characterized by the maximum flexibility, both in regards of delivered feedstock (biomass) and the obtained products. In turn, bio-refineries of the third generation are divided with respect to the applied feedstock and, for example, there are the following refineries: green bio-refinery, complex bio-refinery (called whole crop bio-refinery), ligno-cellulosic and forestry biorefinery, marine biomass bio-refinery etc.[4 ].
Some researchers classify bio-refineries taking into considerations applied technological processes. In this case, the simplest division applies to the two groups of processes: thermochemical processes (in the wider meaning) and biochemical processes [10,11]. Naik et al. [4] distinguish four groups of operations and corresponding unit processes:
? thermo-chemical conversion (in the strict sense, concerning gasification, pyrolysis, liquefaction, combustion)
? biological conversion (anaerobic processing, fermentation, enzymatic conversion)
? chemical conversion (hydrolysis, extraction, conversion under supercritical conditions)
? physical conversion (mechanical extraction, briquetting, distillation).
A simplified description of selected types of bio-refineries
Examples of conventional bio-refineries: sugar factories producing starch, vegetable oil production plants, paper industry plants, biofuels producing plants (such as biodiesel, bioethanol) and others [9]. Green bio-refinery includes treatment of unprocessed (raw, i.e. undried) ?green? plant biomass ? such as grasses, herbs, alfalfa, etc. In this type of bio-refineries the first step in processing is batch feedstock separation as a result of which two distinctive fractions are obtained: the so called fiber-rich press cake (with high content of fibers) and nutrient-rich green juice, (rich in dissolved components for further synthesis) [12, 4, 9].
Complex bio-refinery includes the use of crops in dry or moist form. Crops are used entirely in the state obtained after crops harvesting, and the first step in the process is to separate the grains (about 20% of the whole mass) from the remaining parts of the plant (about 80% of mass) [9]. Application of thermochemical processes in this type of bio-refineries include gasification of a solid part and use of the resulting synthesis gas in a Fischer-Tropsch process [12]. Lignocellulose bio-refineries use feedstock with a high content of lignin, cellulose and hemicellulose, which in practice means use of biomass of forest origin. In the literature, there exists even the concept of ?the forest bio-refinery? [13]. Typically, the first step in the feedstock processing is its separation of the feedstock into these three components: lignin, cellulose and hemicellulose. There are two basic types of separation: acidic separation (usually in the presence of phosphoric acid) and the separation in the presence of ionic liquids. Further conversion of the biomass is a biological treatment (fermentation, enzymatic processes) and process residues are processed in the gasification process [14, 15]. A detailed description of lingo-cellulosic bio-refinery processes (unit processes and research examination of physicochemical characteristics of the feedstock to the process) can be found in the works of Fitz Patrick et al. [14], Limay and Ricke [15] and Zhu Zhuang (energy balance of lingo-cellulosic bio-refinery sugar platform) [16]. A detailed description of the initial stages of forest biomass processing in biorefinery with the analysis of physical and chemical processes that occur during these operations were given by Pedram and YongHao [17] and Zhu [18].
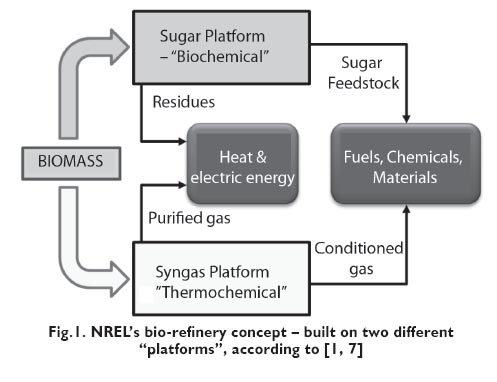
Two-platform type bio-refinery involves a configuration divided into: biochemical platform (also known as sugar platform) and thermochemical platform. This arrangement, proposed by the NREL, is shown in Figure 1. Noticeably, the direct use of biomass in bio-refinery system is concentrated around two main paths of process (Fig.1). The biochemical platform forecasts fermentation or enzymatic processes to convert biomass into a sugars, having 6 carbon atoms in the molecule. The thermochemical platform converts biomass in the process of gasification (to synthesis gas) for further use for chemicals manufacture (for example in Fischer-Tropsch process) or the conversion both to electricity and heat (boiler system) [7]. Recently, a growing interest is observed in the marine biomass use (microalgae and macro-algae [seaweed]) as a feedstock for biorefineries. Jung et al [19] performed an analysis of the potential use of marine biomass as a feedstock to the bio-refinery process. The authors emphasize that the use of marine biomass as a feedstock for bio-refineries solves an important problem in the framework of the so-called debate ?fuel versus food?. In other words, the use of biomass of land origin sometimes generates problems of limited access to land for cultivation and water, which is necessary for irrigating cultivation areas. Cultivation of algae does not create such a high demands for purity of water and is completely neutral, taking into account the use of land for agriculture [20,19]. Microalgae, due to the high content of lipids in their composition are mainly used for the production of biodiesel. For example, Neochloris oleoabundans species contains up to 65% of lipids in the dry mass of algae. A popular species Chlorella vulgaris may contain up to 58% of lipids by weight, and the production capacity of such species is up to 0.96 g/m2 per day cultivation [21].
4. Thermal Bio-refinery
Thermal (thermo-chemical) bio-refinery comprises a complex system, which uses the following processes of biomass thermal conversion: torification (low temperature pyrolysis), pyrolysis, gasification and combustion. Basically there are two types of thermal bio-refinery, depending on the pivotal role of gasification or pyrolysis. In addition to these two main processes, also an important role is played by biomass supply systems for various types of chemical reactors, Dai et al. [22].
4.1. Thermal bio-refinery based on gasification process
The concept of comprehensive thermo-chemical bio-refinery, in which gasification process plays a key role, presented Boerrigter et al. [2]. A conceptual diagram of such a bio-refinery, is shown in Figure 2. In addition to the products obtained at different stages of bio-refinery the concept provides for partial recycling of products (water, ammonium salts, mineral part) into the environment.

Selected partial stages of bio-refinery based on biomass gasification
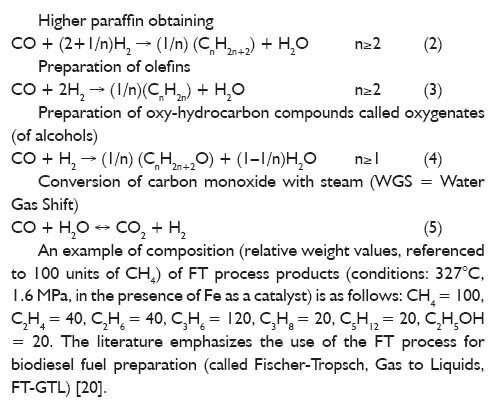
Torification is a process of low-temperature pyrolysis (thermal conversion without presence of oxidant), kept in the range of 200? 300°C; residence time depends on the type of reactor and kind of feedstock (usually 15?30 min). The feedstock particle size for the process is typically about 2 cm, and the rate of heating does not exceed 50°C/min. During the process, biomass loses significant amounts of oxygen and hydrogen compared to organic carbon content (primarily due to moisture evaporation and, to a lesser extent, by the liberation of some amount of volatile organic compounds), which results in an increase of calorific value of solid product of the torification process. For example: wood biomass in the process run at the temperature of 300°C gives a product of calorific value equal to 21 MJ/kg. The content of volatile matter and humidity after the process are in the range of 60?75%, and 0?3%, respectively [23]. The biomass from energy crops (e.g. Miscanthus giganteus or Salix viminalis, the so called Energy Willow) [23, 24] is the most popular raw material for low temperature pyrolysis [torification process] and the products of particular importance for bio-refineries are: furfural, formic acid and acetic acid, which, after appropriate processing are substances with a purity accepted for chemical synthesis [2]. Gasification is a process of thermal conversion, carried out in an atmosphere of insufficient amount of oxidizer (partial oxidation, with excess oxygen ratio ? <1), typically in the range of 800?1200°C. Process gas, whose composition depends on the type of reactor and kind of oxidant, is the main product of the gasification process. When oxygen gasification has taken place, gas calorific value of process gas can reach up to 18 MJ/Nmn 3 [23]. Polish research units have a rich experience in biomass gasification. For example, during the gasification of alder wood chips in a 3-zone fixed bed reactor GazEla (capacity about 60 kWth, Institute for Chemical Processing of Coal (IChPW) in Zabrze) the obtained gas (dry condition) showed a calorific value of about 5 MJ/mn 3 and average composition (volume fractions): CO (25%), CO2 (9.5%), H2 (7.5%), CH4 (2.0%) and N2 (56%) [25, 26]. Tar removal from a process gas can be carried out by a process whose main feature is a scrubber, to which an organic solvent is fed. Tar is separated into three main fractions: heavy tars, light tars and BTX (benzene, toluene, xylene). In water scrubber, inorganic ammonium compounds [formed as a result of HCN to NH3 conversion] are removed. Due to the low content of sulphur in plant biomass, yield of elemental sulphur as a product is not a significant contribution to the economic balance of the bio-refinery. CO2, separation of which is provided in a cycle of thermal bio-refinery, can be used, for example, for greenhouse crops. Cryogenic distillation products, ethylene and acetylene, are an important intermediate in the chemical industry. Methane, obtained as a fraction with a lower boiling point (-162°C at a pressure of 1 bar) can be used as fuel, both in stationary combustion chambers and in the motor vehicle engines [2]. Once all the described operations are carried out, the obtained synthesis gas consists mainly of CO and H2. One way of using synthesis gas is to use it in the process of synthesis gas catalytic conversion (Fischer-Tropsch [FT] synthesis) to obtain liquid hydrocarbons. The most commonly used catalysts are: iron-based, cobalt-based and nickel-based, and depending on the catalysts and process conditions (particularly temperature) a specified selectivity to the desired product is obtained. Chemistry of the FT process involves a lot of reactions; a simplified stoichiometry description is determined by the following equations (based on stoichiometry) [27]:

The remaining syngas or other flammable gases that arise at different stages of the bio-refinery system, are transferred to the conversion system to obtain electrical energy and heat. It is worth to note that there are also other ways to use the bio-refinery byproducts obtained in different generations, such as for example, fuel for boiler. Latkowska et al. [28] conducted research on the use of post-fermentation solid product (called Dried Distillers Grain, DDG) from bio-ethanol production ? as a fuel dedicated to various thermal processes, they say that its co-incineration with coal in existing energetic blocks is one of the best options to recovery of tested material energy. This fuel is characterized by a relatively high calorific value (in the operational state it was 18.6 MJ/kg).
4.2. Thermal bio-refinery based on fast pyrolysis process
Naik et al. [4] have proposed the use of pyrolysis process (in particular the fast pyrolysis process) in different types of bio-refineries, e.g. in thermo-chemical platform of two-platform bio-refinery (see point 2.1, Basic characteristics of bio-refineries). And what is more, biomass pyrolysis is considered as alternative path for bio-refinery ? in view of possibility to apply this technology in existing classic refinery plant. Direct product of liquid fraction during biomass pyrolysis, the socalled bio-oil can be directly used in the refinery. The bio-oil is a mixture whose composition strictly depends on the type of a parent substance, but in the case of forest biomass, the raw material contains about 30% of water, 30% of phenols, 20% of aldehydes and ketones, 15% of alcohols and 5% of other products. Depending on process conditions (mainly heating speed and residence time of the parent substances) three types of pyrolysis are distinguished: conventional pyrolysis, fast pyrolysis and flash pyrolysis.
Conventional pyrolysis includes processes performed at heating speed range 0.1?1 K/s and residence time of biomass in the high temperature zone of the reactor from 45 to 550 s. Normally, unfragmented pieces of wood or other biomass compose a charge. Fast pyrolysis differs significantly from conventional pyrolysis. In this method, a preferred charge is composed of fine fractions of biomass with particle size below 1 mm and process is carried out in the temperature range of 580?980°C at high heating speeds in the range of 10?200 K/s. The residence time of the parent substances in the reaction space is from 0.5 to 10 seconds. Flash pyrolysis carried out in the temperature range of 780?1030°C at very high heating speeds (above 1000 K/s) and short residence times of the parent substances in the reaction space (below 0.5 s). The use of biomass with fine particle size below 0.2 mm is required. Bio-oil is the main product in the process and it can be mixed with fine particles of carbonization product creating so-called biosludge [4]. The biggest portion of liquid fraction of pyrolysis is noted in the case of fast pyrolysis and flash pyrolysis and therefore it is proposed that this process is applied w in the concept of thermal and pyrolithic bio-refinery. After cooling and condensation of liquid products, a dark brown liquid is obtained (the so-called bio-oil) whose calorific value is about a half of calorific value of conventional heating oil. Particular products of fast pyrolysis are the following: 60?75% of bio-oil, 15?25% of carbonization product and 10?20% of uncondensed gas products. Figure 3 shows a simplified flow sheet for fast pyrolysis process whose main product is bio-oil [4,10].
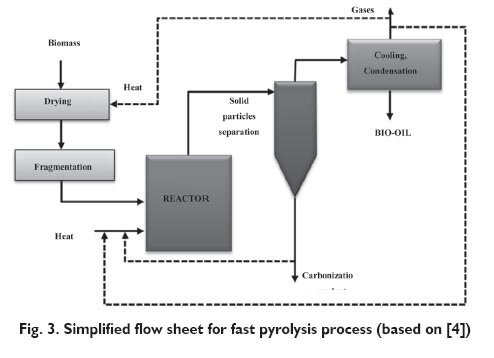
The essence of the fast pyrolysis process consists in intensive heating of biomass charge up to set temperature (usually about 500°C), keeping the charge in the reactor during appropriate residence time and intensive cooling of volatile products for the most efficient obtaining of liquid fractions. The key stage of the process is heat transport to the surface of pyrolysed biomass particles. It is recommended that convective heat-transfer coefficient to reacting particles of biomass in high temperature zone of reactor is at least 500 W/m2K [29]. Bridgwater and Peacocke [30], Venderbosch and Prins [29] and Bridgwater [31] have described various types of pyrolysis plants which work in ?fast process? regime along with basic characteristics of obtained products and possibilities of their application in production of fuels and chemicals.
Properties of bio-oil obtained in fast pyrolysis process are dependent on various conditions. The most important are the following: type of charge and its properties (in particular a moisture content), temperature inside the reactor, the residence time of the parent substances in the reaction space and occurrence of secondary processes (catalytic cracking and thermal cracking). An observed very wide range of coefficient of kinematic viscosity for bio-oil from 11 to 115 mm2/s can prove how strong an influence the above mentioned conditions have on properties of bio-oil. Usually, gross calorific value of bio-oil is below 26 MJ/kg. For comparison, gross calorific value of oils obtained in conventional refining process is about 42?45 MJ/kg. Additionally, bio-oils can absorb up to 35% of water in contrast to traditional petrochemical oils which are insoluble in water [10].
As a product, bio-oil can be used in various processes carried out in order to improve its properties or/and conversion to obtain other products (e.g. methanol). Bridgwater [31] has made a synthetic description of bio-oil processing methods (so-called upgrading). The upgrading methods are divided into three groups: physical, catalytic and other. An example of physical method is separation of bio-oil and solid fraction (ash, carbonization product) by method of hot-vapour filtration which enables one to obtain less than 0.01% w/w solid particles in bio-oil. Other physical method is micro-emulsification of bio-oil with diesel oil (5?95% w/w of bio-oil) in order to use the micro-emulsion as fuel in transport industry or stationary furnaces. Bio-oil conversion processes in the presence of hydrogen and metal catalysts (on platinum, palladium, ruthenium or molybdenum matrix) and cracking in the presence of zeolites have been indicated by the author of [31] as examples of catalytic methods. The essence of the catalytic process in the presence of hydrogen and metal catalysts (so-called hydro-treating process) consists in conversion of hydrocarbon products which contain oxygen (so-called oxygenates). The following methods have been indicated by Bridgwater [31] as other methods of using pyrolysis products: conversion of bio-oil in the presence of water (relatively new approach; using effect of bio-oil solubility in water); cracking in the presence of other catalysts (so-called mild cracking in the presence of ZnO, MgO and other oxides); esterification and intermediate processes; gasification and other methods.
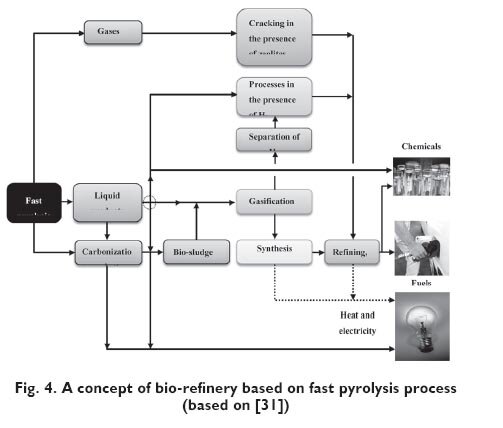
Bridgwater [31] has presented an idea about bio-refinery based on fast pyrolysis process
Figure 4 shows a schematic diagram of bio-refinery, its central pyrolithic part, to be more precise, that can be integrated with bigger system where also biological processes are carried out, mainly fermentation process. Liquid products can be used directly for production of required final products or they can be directed to further processing (e.g. by processes in the presence of hydrogen). Bio-sludge (mixture of some part of liquid products and carbonization product) can be directed to gasification reactor and its products (after appropriate conditioning) can be fed to the synthesis system (e.g. in Fischer-Tropsch process).
5. Bio-refinery in Poland and in the world
The research advancement and development of bio-refineries (pilot-scale installations) in Europe was presented by Mandl [12]. The author presented (along with a short description) the plants in different European countries. The noteworthy bio-refinery is, among others, Austrian bio-refinery (of a ?green? type) in Utzenach, whose main purpose is to obtain lactic acid and amino acids (of the purity required in the food industry) from the ?green juice? (0.2 t/h) derived from grass. One of the most advanced bio-refineries of lingo-cellulosic type is the Norwegian Borregaard plant, located in Sarpsborg [32]. In more than 40-year history of the bio-refinery numerous research and development works were carried out, resulting in the formation of the plant with the wide range of products. Bio-refinery was based on a paper factory, which gradually expanded its product assortment (textile fibres, ethanol, acetic acid…). Until today, the bio-refinery feedstock is spruce-wood, and the plant has four main production lines: cellulose, lignin, ethanol and vanillin. Developmental research focus also on the use of the liquid products of pyrolysis. Among the latest bio-refinery achievements is obtaining cellulose with ultra-thin fibres (called micro-fibrillar cellulose, MFC), which is used to produce lightweight and durable (tensile strength 129?214 MPa) composite materials for automotive industry, manufacture of toys, constructional components of boats and other applications [33]. The first commercial bio-refinery based on fast pyrolysis process is a Dutch plant, which is being built within the EMPYRO project framework (full name ?Polygeneration through pyrolysis: Simultaneous production of oil, process steam, electricity and organic acids? ). The main objective of the project is the construction and demonstration of pyrolysis plant (Bio-refinery 25 MWth), whose products are electricity, process steam and fuel obtained from wood biomass. The project is coordinated by BTG (Biomass Technology Group B.V.), The Netherlands. The project was launched in December 2009 and will be completed in November 2013 [34]. The assumed parameters of the process are: demand for wood biomass 120 t/day, annual oil production 22,500 t , annual electric energy production 6,000 MWh , steam 80,000 t [35, 36].
The growing interest in the bio-refineries topic throughout the world resulted in including this issue within projects implemented in Poland. One of the issues of the Thematic Block 4, named ?Integrated production of gaseous and liquid fuels using biomass conversion methods (Agricultural biogas, Bio-refinery)?, which is implemented within the framework of the National Strategic Program for Research and Development Centre (NCBiR) named ?Advanced technologies of obtaining energy? Task 4 ? ?Development of integrated technology of fuels and energy derived from biomass, agricultural waste and others?, is the technical documentation elaboration and demonstration plant creation for bioethanol production from lingo-cellulosic mass [37]. The research efforts aimed at improving the unit processes that can be part of the whole bio-refinery system are also worth noting. As a result of a joint initiatives of the Institute for Chemical Processing of Coal (IChPW) and the Central Mining Institute (GIG), the Centre for Clean Coal Technologies (CCTW) was established in the Silesia region. The pilot and technical scale plants, located within the Centre, allow for investigation and researching (under pressure and non-pressure conditions) of the gasification, pyrolysis and combustion processes, in which the feedstock is solid fuels (including biomass) [38].
6. Bio-refinery in market conditions
Development of bio-refinery in Poland and other European countries highly depends on trends in the fuel and chemical industry. Costs of bio-refinery operation and investment climate of the industrial decision-makers in Europe are also very important matters. Menrad and others [39] presented the results of a survey conducted among sales representatives of companies from the biofuels, chemical, sugar and forest industry, heat and electrical energy production, starch producers and others (food industry). The structure of the companies researched is as follows: 59% of small and medium enterprises (less than 250 employees), and less than 10% of companies hiring above 10,000 employees. The researches have been conducted in many different European countries, the results have been received from 102 units (Germany 50, France 14, Belgium 12, Austria 2, Portugal 1, Great Britain 1, Finland 5, Sweden 3, Holland 17, Lithuania 2, Hungary 1, not specified in the survey 2). Unfortunately, the research doesn?t include information about Polish companies. Among all the researched companies, 29 belong to the biofuel industry. The survey questions referred to bio-refinery matters and its commercial use (Fig. 5). As can be seen, most of the questioned have a positive attitude towards bio-refineries (from the point of view of the commercial future of the technology). The pollsters have also indicated advantages and challenges concerning bio-refinery. As the main advantages, the questioned included: decrease of import of fossil fuel in its crude form (above 60% of the questioned), reinforcement of local economy (above 55% of the questioned), decrease of negative impact on the environment (above 50% of the questioned), chance to interest new consumer area with the new product (above 50% of the questioned). To the main challenges of bio-refinery have been included: high investment costs (above 40% of the questioned), lack of experience, immature technology (above 35% of the questioned), unstable and changing quality of the feedstock (above 35% of the questioned) and production costs (above 35% of the questioned).

From the point of view of locating of bio-refinery in commercial and market conditions the system, cost and economic analysis are important. Often, there are optimization calculations conducted aiming to maximize the economic potential, EP, i.e., profit excluding the capital costs and operating and maintenance costs [40]. In the literature, there are a few papers of this nature to be found. For instance, Tay and others [41] conducted a system analysis of biorefinery working on the basis of gasification process. The researchers assumed four main types of products: dimethyl ether (DME), fuel from the Fischera-Tropscha process, alcohol solution (except methanol) and methanol. The researchers have been analyzing mainly two cases: of bio-refinery oriented mainly at the DME and the DME and methanol production and they obtained results of economic potential as follows: 43.77 USD/h at the efficiency of 604.29 kg/h of DME and 15.40 USD/h at the efficiency of 100 kg/h of methanol and 564.10 kg/h of DME. The authors have highlighted that the gasification process is going to take place with higher pressure (36 bar) in order to decrease the cost connected to syngas compression for transportation purposes. Ng and others [40] have presented a financial analysis of the integrated bio-refinery oriented at compound synthesis and heat and electrical energy production. The researchers have analysed two case studies of bio-refineries with the feedstock of wood biomass and palm kernel. The key process has been gasification and the products (apart from heat and electrical energy) have been also, similarly to the previous work, the fuel from the FT process, DME and alcohols solution. In case of the bio-refinery based on wood biomass, the economic potential achieved was on the level of 262.2 USD/h at production of 957 kg/h of DME, while in case of the bio-refinery based on the palm kernel (60 t/h of crude feedstock) the economic potential of 182.61 USD/h has been achieved in the manufacture of fibres, pellets and briquettes. Palm kernel shells (PKS) have been directed to the associable designed boiler, to the heat and electrical energy production. Bridgwater [31] has presented a model allowing to estimate the bio-oil production costs in a bio-refinery based on wood or grass pyrolysis. The model calculation results are being presented in the Figure 6.

7. Resume Bio-refineries are of great interest.
It does not change the necessity of a critical view on the current state of the technology development as well as on the accepted directions for further research. It seems that the bio-refineries of the bio-chemical (?sugar?) profile are now at a higher degree of technical and technological development than thermal bio-refineries that are just entering the stage of taking over of pilot and demonstration scale facilities. This is due to the fact that bio-refineries of bio-chemical profile frequently took advantage of the experience gained in the already existing systems (bio-refineries of the first generation), for example sugar factories, distilleries and others. Pyrolysis and gasification processes were initially used for thermal conversion of coal, hence some differences in the development of a biomass refinery technology of bio- and thermotype arise. It should be noted that the systems in which the key role is played by thermo-chemical processes, have many advantages and, despite some delays in the progress of technology, they may compete with the bio-type refinery. It is enough to mention that, for example, the systems based on fast pyrolysis process can be integrated with the systems of refining fossil fuels. Another advantage, in favour of thermo- systems, is the possibility of their application in bio-refineries of bio-chemical type, which is not necessarily obvious in the opposite direction. Additionally, thermal processes are characterized by higher speeds, comparing with e.g. fermentation processes, and do not generate problems associated with a limited biological stability of the feedstock or intermediates. For example, the research on calorific value of post-fermentation broth, DDG, were initiated by problems which prevented the use of this material for fodder production. In theory, this material is well suited for this purpose but in practice the rapidly progressing biological processes (e.g., secondary fermentation, putrefaction, the presence of mould) caused some limitations associated with the use of broth for fodder purposes. This does not mean that thermo- bio-refineries stand in strong opposition to the bio-type systems. These systems may co-exist simultaneously and even be integrated. It should be emphasized that there is a necessity of carrying out researches using pilot plants of these processes that may contribute to the application of thermo-chemical bio-refinery for a commercial scale. The presented cost analyses, despite their usefulness, will not give the full picture of the state of the commercial bio-refinery, unless the full, commercial scale experience in several sites is gained. Only then will a more complete analysis of the costeffectiveness of technology be possible to conduct. Table 1 shows the main outcomes and conclusions related to the progress of thermochemical bio-refinery. The left column shows the rated issue, the middle ? the assessment (using the following criteria, i. e. ?-?, ?+/-? and ?+?) and the right column the substantiation of the assessment. A pilot scale experimental testing and system techno-economic analyses allow for concluding that this application on a commercial scale is likely to be successfully completed by 2030.

Acknowledgements
Financed from the budget of the research task No. 4: ?Developing Integrated Technologies of Fuel and Energy Production from Biomass, Agricultural Wastes and Other Resources? within the framework of the strategic program of research and development: ?Advanced Technologies for Energy Generation? carried by The National Centre of Research and Development.
Literature
1. http://www.nrel.gov/biomass/biorefinery.html 31.01.2013
2. Boerrigter H., Deurwaarder E.P., Bergman P.C.A., Van Paasen S.V.B., Van Ree R.: Thermal bio-refinery; high-efficient integrated production of renewable chemicals,(transportation) fuels, and products from biomass. The 2-nd World Conference and Technology Exhibition on Biomass for Energy, Industry and Climate Protection, Rome, Italy Materiały konferencyjne, 2004
3. Menon V., Rao M.: Trends in bioconversion of lignocellulose: Biofuels, platform chemicals & biorefinery concept. Progress in Energy and Combustion Science 2012, 38, 4, 522?550.
4. Naik S.N., Goud V.V., Rout P.K., Dalai A.K.: Production of first and second generation biofuels: A comprehensive review. Renewable and Sustainable Energy Reviews 2010, 14, 2, 578?597.
5. Kamm B., Gruber P.R., Kamm M.E.: Biorefineries- Industrial Processes and Products. Vol. 1 Wiley ? VCH Verlag GmbH & Co. KGaA 2006,
6. Kamm B., Gruber P.R., Kamm M.E.: Biorefineries- Industrial Processes and Products. Vol. 2. Wiley ? VCH Verlag GmbH & Co. KGaA 2006,
7. Fernando S., Adhikari S., Chandrapal C., Murali N.: Biorefineries: Current Status, Challenges, and Future Direction. Energy & Fuels 2006, 20, 4, .
8. Aresta M., Dibenedetto A., Dumeignil F.: Biorefinery: From Biomass to Chemicals and Fuels. De Gruyter 2012,
9. Ree R.V., Annevelink B. (2007) Status Report Biorefinery 2007. Agrotechnology and Food Sciences Group, Wageningen
10. Demirbas A.: Biorefineries: Current activities and future developments. Energy Conversion and Management 2009, 50, 11, .
11. Fatih Demirbas M.: Biorefineries for biofuel upgrading: A critical review. Applied Energy 2009, 86, Supplement 1, 0, S151-S161.
12. Mandl M.G.: Status of green biorefining in Europe. Biofuels, Bioproducts and Biorefining 2010, 4, 3, 268?274.
13. Moshkelani M., Marinova M., Perrier M., Paris J.: The forest biorefinery and its implementation in the pulp and paper industry: Energy overview. Applied Thermal Engineering 2013, 50, 2, .
14. Fitzpatrick M., Champagne P., Cunningham M.F., Whitney R.A.: A biorefinery processing perspective: Treatment of lignocellulosic materials for the production of value-added products. Bioresource Technology 2010, 101, 23, .
15. Limayem A., Ricke S.C.: Lignocellulosic biomass for bioethanol production: Current perspectives, potential issues and future prospects. Progress in Energy and Combustion Science 2012, 38, 4, 449?467.
16. Zhu J.Y., Zhuang X.S.: Conceptual net energy output for biofuel production from lignocellulosic biomass through biorefining. Progress in Energy and Combustion Science 2012, 38, 4, 583?598.
17. Pedram F., Yonghao N. (2011) Integrated Forest Biorefinery ? Sulfite Process. In: Sustainable Production of Fuels, Chemicals, and Fibers from Forest Biomass, vol 1067. ACS Symposium Series, vol 1067. American Chemical Society, pp 409?441. doi:doi:10.1021/bk-.ch016 10. 1021/bk-.ch016
18. Zhu J.Y. (2011) Physical Pretreatment ? Woody Biomass Size Reduction ? for Forest Bio-refinery. In: Sustainable Production of Fuels, Chemicals, and Fibers from Forest Biomass, vol 1067. ACS Symposium Series, vol 1067. American Chemical Society, pp 89?107. doi:doi:10.1021/bk- .ch004 10.1021/bk-2011-1067.ch004
19. Jung K.A., Lim S.-R., Kim Y., Park J.M.: Potentials of macroalgae as feedstocks for bio-refinery. Bioresource Technology, 0,
20. Nigam P.S., Singh A.: Production of liquid biofuels from renewable resources. Progress in Energy and Combustion Science 2011, 37, 1, 52-68.
21. Mata T.M., Martins A.A., Caetano N.S.: Microalgae for biodiesel production and other applications: A review. Renewable and Sustainable Energy Reviews 2010, 14, 1, 217-232.
22. Dai J., Cui H., Grace J.R.: Biomass feeding for thermochemical reactors. Progress in Energy and Combustion Science 2012, 38, 5, 716-736.
23. Robbins M.P., Evans G., Valentine J., Donnison I.S., Allison G.G.: New opportunities for the exploitation of energy crops by thermochemical conversion in Northern Europe and the UK. Progress in Energy and Combustion Science 2012, 38, 2, 138-155.
24. Kopczyński M.: Toryfikacja wierzby energetycznej. Energetyka cieplna i zawodowa 2012, 2, 39?44.
25. Sobolewski A., Kotowicz J., Iluk T., Matuszek K.: Badania eksperymentalne zgazowania biomasy pod kątem wykorzystania gazu procesowego w układzie kogeneracji. Przemysł Chemiczny 2010, 89, 6, 794?798.
26. Sobolewski A., Kotowicz J., Iluk T., Matuszek K.: Wpływ rodzaju biomasy na parametry pracy generatora gazu ze złożem stałym. Rynek Energii 2009, 3, 82, 53?58.
27. Joźwiak W.K., Lewicki A., Kwintal-Ogórek P., Michalak A., Nowosielska M.: Kataliza w syntezie Fischera-Tropscha. 2010,
28. Latkowska B., Fitko H., Stelmach S.: Ocena właściwości paliwowych ubocznego produktu z produkcji bioetanolu. Inżynieria ekologiczna 2011, 25, 222?230.
29. Venderbosch R.H., Prins W.: Fast pyrolysis technology development. Biofuels, Bioproducts and Biorefining 2010, 4, 2, 178-208.
30. Bridgwater A.V., Peacocke G.V.C.: Fast pyrolysis processes for biomass. Renewable and Sustainable Energy Reviews 2000, 4, 1, 1-73.
31. Bridgwater A.V.: Review of fast pyrolysis of biomass and product upgrading. Biomass and Bioenergy 2012, 38, 0, 68-94.
32. R?dsrud G., Lersch M., Sjöde A.: History and future of world?s most advanced biorefinery in operation. Biomass and Bioenergy 2012, 46, 0, 46-59.
33. Spence K.L., Venditti R.A., Habibi Y., Rojas O.J., Pawlak J.J.: The effect of chemical composition on microfibrillar cellulose films from wood pulps: Mechanical processing and physical properties. Bioresource Technology 2010, 101, 15, 5961-5968.
34. http://www.empyroproject.eu/ 14.02.2013
35. http://www.btg-btl.com/uploads/documents/2010-12%20Leaflet%20 Empyro%20%28Eng%29.pdf 11.02.2013
36. http://www.btg-btl.com/index.php?id=76&rid=47&r=oilapplicati on 15.02.2013
37. http://www.strateg-z4.imp.gda.pl/projekt-blok-4.html 13.02.2013
38. Lajnert R., Latkowska B.: Potencjał badawczy instalacji technologicznych Centrum Czystych Technologii Węglowych (CCTW) w Zabrzu. Przemysł Chemiczny 2013 (w druku), 92, 2,
39. Menrad K., Klein A., Kurka S.: Interest of industrial actors in biorefinery concepts in Europe. Biofuels, Bioproducts and Biorefining 2009, 3, 3, 384?394.
40. Ng R.T.L., Tay D.H.S., Ng D.K.S.: Simultaneous Process Synthesis, Heat and Power Integration in a Sustainable Integrated Bio-refinery. Energy & Fuels 2012, 26, 12, 7316-7330.
41. Tay D.H.S., Kheireddine H., Ng D.K.S., El-Halwagi M.M., Tan R.R.: Conceptual Synthesis of Gasification-Based Biorefineries Using Thermodynamic Equilibrium Optimization Models. Industrial & Engineering Chemistry Research 2011, 50, 18, 10681-10695.
Janusz LASEK ? Ph.D. Eng., was born on 28th January 1979 in Katowice. He graduated from the Faculty of Materials Engineering and Metallurgy of the Silesian University of Technology in Gliwice (2004). He obtained his doctoral degree (distinction) in metallurgy in 2009 at the Silesian University of Technology and after that he was post-doctoral researcher in the Department of Chemical Engineering of National Taiwan University. The research was related to the photo-catalytic removal of NOx. Since 2010, he has been employed in the Institute of Chemical Processing of Coal as assistant professor (adjunct). He is the author of two sections in monographs, 11 scientific papers and presented scientific research during 7 national and international conferences. Scientific area: conversion of NOx during combustion, DeNOx processes, pressurized oxy-fuel combustion of solid fuels, application of marine biomass as energy resource e-mail: ; phone: + ext. 328
Aleksander SOBOLEWSKI ? Ph.D. Eng., was born on 11th November 1962 in Częstochowa. He graduated from the Department of Chemistry of the Silesian University of Technology in Gliwice (1986) major in chemical engineering. He got his doctoral dissertation in chemical engineering in 1993 at the Silesian University of Technology. Since 1987, he has been employed at the Institute for Chemical Processing of Coal in Zabrze, successively performing the following functions: assistant, assistant professor, department manager and director, and since 2004 – Research and Development Deputy Director of the Institute. Dr. Sobolewski has published several monographs, over 150 scientific papers (in Polish, English, German and Russian) and developed 15 patents. Throughout his entire professional career, he has been closely collaborating with the industry, particularly with coking, power engineering and chemical plants. His expertise also comprises management of research teams and projects (both domestic and international ones) and implementations. e-mail: ; phone: +
