Zuzanna WITKOWSKA, Katarzyna CHOJNACKA, Mateusz SAMORAJ, Łukasz TUHY ?Institute of Inorganic Technology and Mineral Fertilizers, Faculty of Chemistry, Wroclaw University of Technology, Poland
Please cite as: CHEMIK 2013, 67, 7, 642?647
Abstract:
The aim was to develop a new generation feed additive with copper using the biosorption method. The process was conducted in a fixed-bed reactor with capacity of 2.5 dm3. Soybean meal constituted the matrix for microelement ions. The bed was enriched by pouring the solution with Cu(II) ions at concentration of 300 mg/dm3 from the top of the column. The process was conducted for 6 hours. The adsorption kinetics was described using Yoon and Nelson kinetic model. The analysis showed that after the first and the second hour, the degree of ion binding was 97.3 and 85.9%, respectively. In the next hours of the process the degree of ion biding was much lower and was 53.4, 33.2, 20.9 and 14.3% after third, fourth, fifth and sixth hour, respectively.
Keywords: bio-sorption, feed additives, fixed-bed reactor, microelements, soybean meal
A new generation of feed additive with copper using the biosorption method in a fixed-bed reactor was produces. Soybean meal constituted the matrix for microelement ions. Biosorption is a process of reversible ion binding from the solution to functional groups occurring on the surface of the non-living biomass [1]. The process is spontaneous, involving simultaneously a few mechanisms. It has been showed in several papers that the dominant mechanism is ion exchange, during which alkali metals and alkaline earth metals ions spontaneously bind with the biomass are released to the solution while the ions of given metal takes their places [1, 2]. The remaining mechanisms of biosorption are for example microprecipitation and adsorption on the surface of the sorbent, but their contribution in the process is much smaller [2, 3]. Biosorption is widely described in the literature. Studies of the process are conducted mainly in terms of the removal of heavy metal ions and dyes from the solutions [4, 5]. Ions are bound with the biomass efficiently and reversibly, therefore the ions and particles contaminating the solution can be removed and the biomass regenerated for the reuse. Additionally, if the ions removed from the solution are valuable, they can be retrieved, for example with the use of the electrolysis. Usually, the regenerative ability of biological materials does not exceed a few cycles [3], and the spent biomass is a waste that requires disposal.
Recently, a new application of biosorption has been proposed ? not as a method of heavy metal ions removal but as a method of biomass enrichment in desirable elements [6÷8]. A new concept is based on the assumption that the biomass to which ions were bound is the final product, while the solution after the biosorption is a waste water that needs to be discharged and treated [3]. New insight into the biosorption process could be applied in the search for new source of microelements for animals [6,7]. This problem is particularly important in relation to modern farming in which animals do not have access to natural sources of micronutrients, such as grasses and herbs growing on the pasture, and the demand for particular substances is covered entirely by using specially prepared feed premixes [9]. Currently commercially available formulations with microelements for feeds have a number of disadvantages, including low bioavailability and high toxicity (inorganic salts) and very high price (organic chelates), which prompts the search for new ways to provide animals with adequate doses of micronutrients in the nontoxic and highly available form [3].
If the biological material, edible for animals and approved by obligatory law as a feed ingredient (i.e. barley, soy, wheat, rapeseed meal) would be applied in the process of biosorption, and metal cations would be derived from inorganic salts approved as feed supplements (i.e. MnSO4?H2O, ZnSO4?H2O) [10÷12], innovative biological feed supplements with microelements can be obtained. The ion of given microelement is bound with the plant cell surface, therefore this form or microelement should be regarded as natural by the organism of animal. As a biomass, any material of biological origin that is characterized by good biosorption ability could be used, but if the traditional feed material would be applied (like soybean meal), no ingredients that could change the general tastiness of the feed are introduced, which could occur for example when using enriched seaweeds [6, 7]. So far, several papers on the production of new biological feed additives with microelements by biosorption method have been published. Also, the effect of new preparations on animals health and performance of laying hens and pigs was studied [6÷8, 13]. In these works for the preparation of new supplements, a mixing reactor was used, which provides a high biosorption capacity of the biomass, but a low productivity. The aim of this paper was to discuss a production of new biological supplements with Cu(II) in the reactor column. The productivity of this reactor is many times greater than the mixing reactor, and obtained biosorption capacities are high enough to use enriched biomass as a supplement with microelements for animals.
Materials and Methods
Soybean meal (Vetos, Zębowice, Poland) was enriched with Cu(II) by biosorption method, with the use of CuSO4?5H2O (POCH, Gliwice, Poland). The process was carried out in a continuous mode in a fixed-bed reactor with capacity of 2.5 dm3. The column packing constituted 400 g of soybean meal. The bed was enriched by entering the solution with Cu(II) ions at concentration of 300 mg/dm3 from the top of the column. The flow rate of the solution through the bed was 83 cm3/min. The process was carried out at 20°C for 6 h. The samples of the solution outflowing from the column were taken every hour. The process was carried out in a deionised water, which pH was adjusted to 5.0 with 0.1 mol?l-1 NaOH/HCl (POCH, Gliwice, Poland). pH was measured with pH meter equipped with an electrode (InLab413) with the compensation of temperature (Mettler-Toledo Seven Multi; Greifensee, Switzerland). Samples of the biomass before and after the biosorption were taken for elemental analysis. Enriched biomass was air-dried for 48 h. For elemental analysis, samples of natural and enriched biomass were taken. The samples were homogenized according to Polish norm PN-90/R-64769 and mineralized [14]. Also, samples of the solution out flowing from the column were taken for the analysis. Inductively Coupled Plasma-Optical Emission Spectrometer with ultrasonic nebulizer (Varian VISTA-MPX ICP-OES, Victoria, Australia) was used to determine the content of metal ions in all samples in the Chemical Laboratory of Multielemental Analysis at Wrocław University of Technology, which is accredited by ILAC-MRA and Polish Centre for Accreditation (Nr AB 969) [14].
Results and Discussion
New biological feed additive with Cu(II) was produced by biosorption method in fixed-bed reactor. The general scheme of the process is presented in Figure1.

The matrix for microelement ions constituted soybean meal, the common component of feeds for livestock. Biosorption was carried out in the continuous mode. Elemental analysis of the natural and enriched biomass showed significant increase of Cu(II) ions in soybean meal after biosorption process. Cu(II) content in natural biomass was 0.0640 mg/g, while
after biosorption was 15.7 mg/g (Fig. 2). Obtained preparation contained 246 times more Cu(II) ions than natural soybean meal.
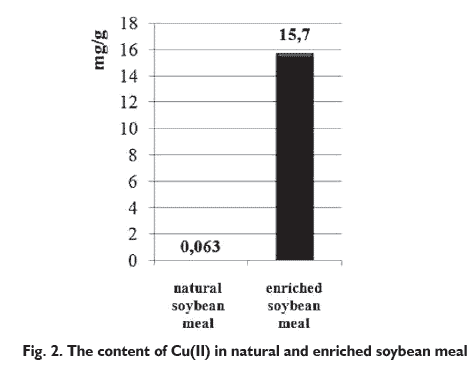
In order to use enriched soybean meal as a feed supplement with copper for laying hens or pigs, the preparation should be added in such amount that covers the need of the animal for this element. According to Standards in Hens Feeding, lower limit for copper supplementation of hens is 5 mg/kg [9], while Standards in Pigs Feeding state that lower limit for Cu(II) for finishers is 20 mg/kg [15]. To cover the requirement of laying hens for this element, 0.332 g of the preparation should be added to each kilogram of feed. In the case of finishers, the amount of new supplement added to feed should be 1.29 g per each kilogram of feed. These amounts are small enough to make the use of new supplement cost effective from the economic point of view [13], yet large enough to distribute the preparation throughout the mixture. Mathematical models that are most frequently used for description of kinetic of biosorption in fixed bed are Yoon and Nelson, Thomas and Clark models [16, 17]. In our study, Yoon and Nelson model was chosen to describe the kinetics of the process (Eq.1.) [16]:
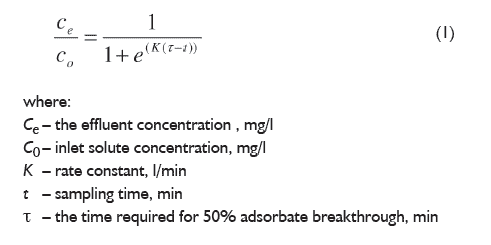
This model is less complicated than other models and also does not require detailed data concerning the characteristics of sorbate, the type of biosorbent as well as the physical properties of the bed [16, 17]. This model is based on the assumption that the rate of decrease in the probability of adsorption for each adsorbate molecule is proportional to the probability of adsorbate adsorption as well as the probability of adsorbate breakthrough on the adsorbent. Previous studies have shown that this model could be applied for description of biosorption in fixed bed reactor [16]. The ICP-OES analysis of samples taken at the outlet of the column showed that the Cu(II) concentration was the lowest in the samples collected at the beginning of the process, and with time the concentration of copper ions in the solution leaving the column increased. After the first and the second hour of the biosorption, the degree of ion binding was 97.3 and 85.9%, respectively. In the next hours of the process the degree of ion biding was much lower and was 53.4, 33.2, 20.9 and 14.3% after third, fourth, fifth and sixth hour, respectively. This indicates that the sorbent was binding Cu(II) ions the most effectively during the first two hours of the process.
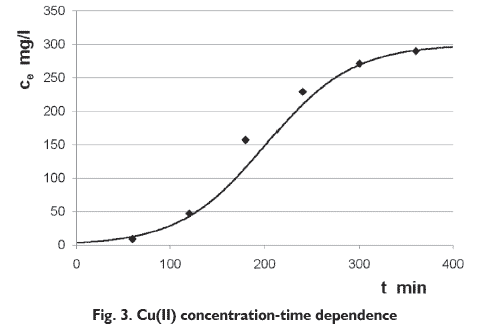
Young and Nelson kinetic model describes well the breakthrough curve biosorption of Cu(II) ions by the soybean meal. The model parameters of kinetic were determined by linearization method described in the work of Nwabanne and Igbokwe [16]. Calculated time required for 50% adsorbate breakthrough (?) was 201 min, while the rate constant (K) was 0.022 l/min. Results obtained by elemental analysis of samples indicate that biosorption process in the bed-fixed reactor is preferably carried out for the first 3 hours. Longer carrying out of the process causes more ions to flow out of the reactor than binding with the biomass. According to Polish law, to discharge the solution flowing out from the column to the sewage, the Cu(II) concentration should not exceed 0.5 mg/dm3 [18]. In order to reduce the concentration to this value, the solution should be collected and the second biosorption on a new bed should be performed to remove the residues of copper ions.
Summary
A new generation feed additive with copper using the biosorption method was produced in the fixed-bed reactor. Soybean meal can be the biological carrier of Cu(II) ions in livestock diet. Elemental analysis of samples showed that the content of Cu(II) in the biomass increased 246 times. 0.332 and 1.29 g of the preparation should be added to each kilogram of the feed to cover the requirement of laying hens and pigs, respectively, for this element. Young and Nelson kinetic model was chosen to describe the breakthrough curve biosorption of Cu(II) ions by the soybean meal. The studies showed that in the samples taken from the outlet of the reactor, after the first and the second hour the degree of ions binding was 97.3 and 85.9%, respectively. In the next hours of the process the degree of ions biding was much lower and was 53.4, 33.2, 20.9 and 14.3% after third, fourth, fifth and sixth hour, respectively. The process is preferably carried out for first 3 hours, since the longer carrying of the biosorption causes that more ions flows out from the reactor than is bound with the soybean meal.
Translation into English by the Author
Acknowledgements
The study was financed by the National Centre for Research and Development nr NR05-0014-10.
Literature
1. Davis T.A., Volesky B., Mucci A.: A review of the biochemistry of heavy metal biosorption by brown algae. Water Research 2003, 37, 4311.
2. Volesky, B.W.: Biosorption of heavy metals ? Biosorption and biosorbents. Florida, CRC press, 3?5, 1990.
3. Chojnacka K. . Biosorption and bioaccumulation ? the prospects of practical applications. Environment International 2010, 36, 299?307
4. Volesky B., Prasetyo V.: Cadmium removal in a biosorption column. Biotechnology and Bioengineering 1994, 43, 1010.
5. Witek-Krowiak A., Szafran R.G., Modelski S.: Biosorption of heavy metals from aqueous solutions onto peanut shell as a low-cost biosorbent. Desalination 2011, 265, 126.
6. Michalak, I.: The new generation of biological feed additives with microelements on the basis of macroalgae, PhD Thesis, Wroclaw University of Technology, Poland, 2009.
7. Zielińska A.: Elaboration of production process of new generation of mineral feed additives based on microalgae biomass. PhD Thesis, Wroclaw University of Technology, Poland, 2010.
8. Chojnacka K.: Biosorption and bioaccumulation of microelements by Riccia fluitans in single and multi-metal system. Bioresource Technology 2007, 98, 2919.
9. Smulikowska, S., Rutkowski, A.: Standards in hens feeding, 4th edn., Instytut Fizjologii i Żywienia Zwierząt PAN, Jabłonna, Poland., 2005. (in Polish)
10. Announcement of the Minister of Agriculture and Rural Development, 22th November 2002, Journal of Laws, 2002, No. 204, Item 1726.
11. Announcement of the Minister of Agriculture and Rural Development, 19th January 2005, Journal of Laws, No. 16, Item 137.
12. European Commision, Directive No. 1292/2005, 5th August 2005, Official Journal 2005, 205, 3.
13. Witkowska Z., Chojnacka K., Zielińska A., Zdunek M., Przemysł Chemiczny, 2011, 90, 1069.
14. Michalak I., Chojnacka K.: Bezściekowa technologia produkcji dodatków paszowych z mikroelementami na bazie makroalgi Pithophora varia Wille. Przemysł Chemiczny 2009, 88, 512.
15. Standards in Pigs Feeding. Nutrive Value of Feeds. Institute of Physiology and Animal Feeding, PAN, Omnitech Press, Warszawa, Poland, 1993.
16. Nwabanne J.T., Igbokwe P.K, International Journal of Applied Science and Technology, 2012, 5, 106.
17. Ghribi A., Chlendi M., Modeling of Fixed Bed Adsorption: Application to the Adsorption of an Organic Dye, 2011, 4, 161.
18. Act dated 18th July, 2001 – Water Law, Act on Collective Water Supply and
19. Collective Wastewater Piping Off dated 7th June, 2001, Journal of Laws, No. 123, item 858, in Polish.
Zuzanna WITKOWSKA ? M.Sc., graduated from the Faculty of Chemistry of the Wrocław University of Technology (2009). She is a PhD student at the Institute of Inorganic Technology and Mineral Fertilizers (Agriculture Chemistry Unit) of the Wrocław University of Technology. Specialty ? chemical technology.
Katarzyna CHOJNACKA ? (Sc.D., Eng.), Professor, graduated from the Faculty of Chemistry of the Wrocław University of Technology (1999). In 2003, she obtained the degree of PhD in technical sciences at the Institute of Chemical Engineering and Heat Devices of the Wrocław University of Technology, while in 2008 ? the post-doctoral degree (doctor habilitatus) at the Faculty of Chemistry of the Wrocław University of Technology. Since 2009, she has worked as professor extraordinarius at the Institute of Inorganic Technology and Mineral Fertilizers of the Wrocław University of Technology. In 2012, she became a professor of technical sciences in the discipline of chemical technology. Specialty ? biological technologies.
Mateusz SAMORAJ ? M.Sc., graduated from the Faculty of Chemistry of the Wrocław University of Technology (2012). He is a PhD student at the Institute of Inorganic Technology and Mineral Fertilizers of the Wrocław University of Technology. Specialty ? chemical technology.
Łukasz TUHY ? M.Sc., graduated from the Faculty of Chemistry of the Wrocław University of Technology (2010). He is a PhD student at the Institute of Inorganic Technology and Mineral Fertilizers of the Wrocław University of Technology. Specialty ? chemical technology.
