Sorption of heavy metal complexes with MGDA on the macroporous anion exchanger Purolite A830
Justyna JACHUŁA, Zbigniew HUBICKI ? Department of Inorganic Chemistry, Maria Curie-Skłodowska University, Lublin, Poland
Please cite as: CHEMIK 2013, 67, 8, 693?700
Abstract:
The aim of the paper is to present the research on sorption of heavy metals Cu(II), Zn(II), Cd(II) and Pb(II) with methylglycinediacetic acid (MGDA) on the weakly, basic, – acrylic anion exchanger Purolite A830. Sorption depends on such factors as: chase contact time, pH, concentration, temperature and the presence of other ions. The obtained data are the basis for kinetic and equilibrium analyses. The FT-IR spectra and AFM Picture for the anion exchanger Purolite A830 before and after the sorption of Zn(II)-MGDA complexes are very helpful for explanation.
Keywords: Purolite A830, heavy metals, chelating agents, sorption, MGDA
Introduction
Compounds of heavy metals, particularly of lead, zinc and cadmium as well as copper, arsenic and chromium are noxious substances getting into the environment [1]. These metals and their combinations can be one of the most dangerous forms of environment contamination. This is the world- wide problem resulting from the industrial development [2]. The main sources of water contamination with heavy metals are the plants dealing with mining, processing, enrichment and recovery of metals and their compounds. Significant share is connected with the factories which use heavy metals in technological processes among others, in production of paper, fertilizers, in foundries and steel plants, in car and aviation industries [3]. Due to migration and cumulation, plants, animals and indirectly humans are exposed to toxic action of heavy metals [4]. Since chelating agents of new generation were commercially available, the idea has aroused to combine their application with the studies of heavy metal ions removal from sewages. Due to the ability of formation of anion complexes between heavy metal ions and chelating agents the combination of this type can be removed by means of sorption methods using many anion exchangers or chelating ion exchangers. The proposed method for purification of sewages from heavy metal ions is simple and effective. Moreover, it does not give dangerous side products. The above mentioned complex ones are characterized by good biodegradability and what is more, they are not toxic and do not create additional threat for the environment. So far few papers have been Publisher on sorption of heavy metal ions on anion exchangers in the presence of complexing agents. One of them is on the studies by Nelson et al. about alkali metals as well as Mn(II), Co(II), Ni(II) and Zn(II) sorption in presence of EDTA on the anion exchanger Dowex 1×4 [5]. The studies of applying the chelating agents such as EDTA, NTA or citric acid are carried out also by Bolto, Clifford and Dudzińska [6]. They proved that in the case of sorption of the [Pb(edta)]2- and [Cd(edta)]2- complexes, polyacrylic anion exchangers are characterized by greater effectiveness than those of polystyrene matrix. Juang and co-workers proved that it is possible to apply Amberlite IRA 400 in Cu(II) sorption in the presence of EDTA [7].
Experimental
Materials and methods
There was studied the effect of physico-chemical parameters among others, chase contact time pH, concentration, temperature as well as the presence of other ions on sorption of Cu(II), Zn(II), Cd(II) and Pb(II) complexes with methylglycinediacetic acid . Sodium salt of the acid in question whose commercial name is Trilon M was used in the studies [8]. Acrylic anion exchanger Purolite A830 produced by the American firm the Purolite Company was used in sorption. It is a macro-porous anion exchange resin of high capacity. The acrylic matrix is good for efficient removal of both mineral and organic acids. Its macro-porous construction gives very good mechanical strength and resistance to osmotic shock. Purolite A830 is widely applied for removal of sulfates from sea water and for neutralization of effluents [9]. Table 1 presents some physicochemical properties of the ion exchanger in question.

Static examination was made in 100 cm3 cone flasks filled with 20 cm3 of the initial aqueous phase and 0.2 g of the weighted ion exchanger. Then they were shaker in the mechanical shaker ELPHINE 357. The M(II) ions content in the raffinate was determined by means of the AAS method using a spectrometer SpectrAA 240 FZ produced by the Varian company. The sorption percentage ? %S of M(II) ions in the presence of MGDA on the anion exchanger Purolite A830 and the removal coefficient Kd [l/g] were calculated from the relations:

Kinetic parameters of sorption of Cu(II), Zn(II), Cd(II) and Pb(II) complexes with MGDA were determined from the following Lagergrena relations [10]:

The Maxima sorption capacities qo [mg/g] and the Langmuir constant b [dm3/mg] were determined from the linear dependence of the Langmuir isotherm:

Results and discussion
In terms of following studies influence of pH of the solution of the Cu(II), Zn(II), Cd(II) and Pb(II) complex with methylglycinediacetic acid (MGDA) on effectiveness of sorption on the anion exchanger Purolite A830 was verified. (Fig. 1). In the case of Cu(II), Zn(II)
ions sorption in the presence of methylglycinediacetic acid, pH does not affect significantly the sorption capacity of the anion exchanger Purolite A830. Maximal sorption capacities for these ions are found at acidic pH and are 34.43 mg/g for Cu(II) ions and 30.27 mg/g for Zn(II) ions. The greatest sorption effectiveness of Cd(II) complexes is achieved on this anion exchanger in the pH range 7?9. The increase in pH from 6 to 11 increases removal efficiency of the Pb(II)-MGDA complexes.

The analysis of kinetics of Cu(II), Zn(II), Cd(II) and Pb(II) ions in the presence of MGDA on the anion exchanger Purolite A830 shows that with the passage of phase contact time, the sorption capacity of the ion exchanger increases. In the case of Zn(II)-MGDA and Pb(II)-MGDA complexes, the equilibrium was reached after the chase contact time about 20 minutes and for the other considered metal complexes the equilibrium was established after 60 minutes. After 180 minutes the sorption on Purolite A830 was as high as 61.61% for the Cu(II) ions complexes, 54.91% for the Zn(II) ions complexes, 54.82% for the Cd(II) ions complexes and 23.33% for the Pb(II) ions complexes. The values of the removal coefficient are 0.160, 0.122, 0.121, 0.020 for the Cu(II)-MGDA, Zn(II)- MGDA, Cd(II)-MGDA and Pb(II)-MGDA complexes respectively.
The initial concentration of the metals complex solution was 0.007 mol/dm3.
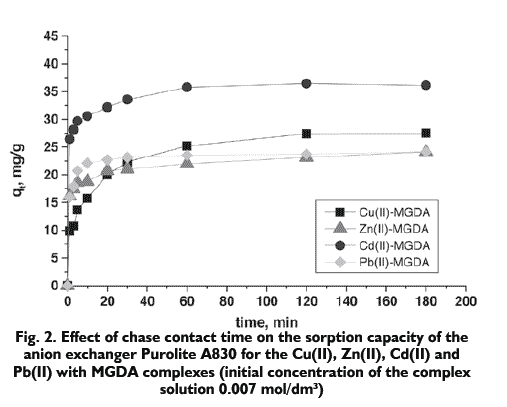
Characteristic kinetic parameters of sorption of the heavy metals Cu(II), Zn(II), Cd(II) and Pb(II) with MGDA complexes were determined from the Lagergren dependences described by equations 3 and 4 (Tab. 2). The correlation coefficient values 0.994?0.999 indicates that the systems in question can be described by the second order kinetic equation and the calculated sorption capacities are close to the experimental data.

Obtained results are in good agreement with literature data, which concerned about usage of different sorbents in sorption of heavy metal ions. The works of Kołodyńska prove that it is possible to apply the anion exchanger Amberlite IRA 458, Amberlite IRA 958 and Amberlite IRA 67 for Co(II) and Ni(II) removal in the presence of IDS. The mentioned sorption process followed the pseudo second order reversible kinetics [12]. Lin and Juang showed that the removal of Cu(II) and Zn(II) ions from water solutions
by means of chelating ion exchangers Chelex 100 and Amberlite IRC 748 can be described according to the equation type of the pseudo second order [13]. In paper [14] on removal Pb(II), Cd(II), Cu(II) and Ni(II) ions by means of zeolites, it was shown that the second order kinetic equation was selected as the most adequate to fitting experimental data.
The maximal sorption capacities (Table 3) for the ion exchanger Purolite A830 for Cu(II), Zn(II), Cd(II) and Pb(II) ions determined based on the linear dependence of the Langmuir isotherm (Fig. 3a) are 33.67 mg/g, 44.84 mg/g, 84.56 mg/g, 28.00 mg/g respectively.

The applicability of the anion exchange in the sorption of the heavy metals and MGDA complexes was determined using the separation coefficient RL described by the reaction [12]:

The value RL>1 is disadvantageous for sorption, RL=1 indicates the linear character, 0 < RL <1 is advantageous and RL=0 indicates the irreversible character of sorption. The value RL for the studies sorption system using the anion exchanger Purolite A830 is 0.71 for the Cu(II)- MGDA complexes, 0.70 for the Zn(II)-MGDA complexes, 0.83 for the Cd(II)-MGDA complexes and 0.69 for the Pb(II)-MGDA complexes. The linear dependence ln qe vs ?2 allows to determine the constans from the Dubinin-Radushkevich isotherm (Fig. 3b). The sorption energy E, described by the equation: E=-(2?)-0.5, is used to determine the sorption mechanism. If this value is between 8 and 16 kJ/mol, it is possible to explain the sorption mechanism by means of ion exchange [13]. The calculated values E (Tab. 3) for the sorption of Cu(II), Zn(II), Cd(II) and Pb(II) in the presence of MGDA on the acrylic anion exchanger Purolite A830 are in the presented range.

The effect of salt on sorption of the M(II)-MGDA complexes on Purolite A830 was also studied. In the research there were used 0.1 mol/dm3 NaCl, Na2SO4 and NaNO3 solutions (Fig. 4). The Cu(II), Cd(II) and Pb(II) ions removal decreased by about 2.3%, 4.2% and 56.2% respectively in the presence of the Cl- ions. In the presence of SO4
2- ions the sorption efficiency of Zn(II)-MGDA and Pb(II)-MGDA complexes decreased by 5.9% and 44.7% respectively.

The influence of temperature on sorption of M(II)-MGDA complexes was studied with the constant initial concentration of 0.001 mol/dm3. As it can be seen on Figure 5, the equilibrium sorption capacity of the Purolite A830 increased when the temperature of the complex solution increased from 293 K to 323 K during the phase contact time. The enhancement in adsorption with temperature may be attributed to the increase in the porosity and in the total pore volume of the anion exchangers.

The FT-IR spectrum (Fig. 6) show characteristic bands for stretching vibrations of the groups ?COO- at about 1611cm-1 and 1401cm-1 (nas(COO-) and ns(COO-)) after the sorption of Zn(II)-MGDA complexes on the anion exchanger Purolite A830. The carboxylate groups are connected with the presence of the anion complex Zn(mgda)? on the studied anion exchanger. The bands at about 3400 cm-1 connected with the deformation vibrations of O?H bond indicate the presence of water in the ion exchanger.

Figure 7 presents the surfaces morphology of Purolite A830 before and after the sorption process of Zn(II)-MGDA complexes. It is noticed that after the sorption process their surface was dense in comparison with the ones not being in contact with metal ions.

Conclusions
The possibility of using the weakly basic anion exchanger Purolite A830 of high capacity in the sorption of heavy metal ions: Cu(II), Zn(II), Cd(II) and Pb(II). This process is possible owning to the presence of methylglycinediacetic acid (MGDA), which forms anion complexes with M(II) ions. This sorption method is affected by many parameters, among others, phase contact time, initial concentration of complex solution and other ions present in the solution. The increase in sorption efficiency with the increasing
pH values from 6 to 11 was observed only for the Cd(II)-MGDA and Pb(II)-MGDA complexes. The proposed method of sewages purification from heavy metal ions is simple and effective and does not generate hazardous side effect. Because of the ability to form anion complexes, this type of combination can be removed by means of sorption methods using a wide range of anion exchangers or chelating ion exchangers.
Literature
1. Pehlivan E., Altun T..: Ion-exchange of Pb2+, Cu2+, Zn2+, Cd2+, and Ni2+ ions from aqueous solution by Lewatit CNP 80. Journal of Hazardous Materials 2007, 140, 307.
2. Bielański A.: Podstawy chemii nieorganicznej, PWN, Warszawa 2010.
3. Kołodyńska D.: Application of strongly basic anion exchangers for removal of heavy metal ions in the presence of green chelating agent. Chemical Engineering Journal 2011, 168, 994?1007.
4. Florczyk H., Gołowin S.: Metale ciężkie w wodach powierzchniowych płynących Polski, Informator Dolnośląskiego Oddziału PZITS, Wrocław 8/1980.
5. Nelson, F.; Day, R.A. Jr; Kraus K.A.: Anion exchange studies-XXX a number of elements in ethylenediaminetetraacetic acid solutions. Journal of Inorganic and Nuclear Chemistry 1961, 15,140?150.
6. Dudzinska, M.R.; Clifford, D.A.: Anion exchange studies of lead-EDTA complexes. Reactive Polymers (1991/1992), 16, 71?80.
7. Juang, R.S.; Lin, S.H.; Wang, T.Y.: Removal of metal ions from the complexed solutions in fixed bed using a strong acid ion exchange resin. Chemosphere
2003, 53, .
8. Broszura informacyjna firmy BASF 2003.
9. Broszura informacyjna The Purolite Company USA 2011.
10. Dizge, N.; Keskinler, B.; Barlas, H.: Sorption of Ni(II) ions from aqueous solution by Lewatit cation-exchange resin. Journal of Hazardous Materials 2009, 167, 915?926.
11. Jachuła, J.; Kołodyńska, D.; Hubicki, Z.: Sorption of Cu(II) and Ni(II) ions in the presence of the methylglycinediacetic acid by microporous ion exchangers and sorbents from aqueous solutions. Central European Journal of Chemistry 2010, 9, 540?547.
12. Kołodynska, D.: Polyacrylate anion exchangers in sorption of heavy metal ions with the biodegradable complexing agent. Chemical Engineering Journal 2009, 150, 280?288.
13. Lin, L.Ch., Juang, R.S.: Ion-exchange kinetics of Cu(II) and Zn(II) from aqueous solution with two chelating resins. Chemical Engineering Journal 2007, 132, 205?213.
14. Sprynskyy, M., Buszewski, B., Terzyk, A.P., Namieśnik, J.: Study of the selection mechanism of heavy metal (Pb2+, Cu2+, Ni2+ and Cd2+) adsorption on clinoptilolite. Journal of Colloid and Interface Science 2006, 304, 21?28.
15. Langmuir, I.: The constitution and fundamental properties of solids and liquids. Journal of the American Chemical Society 1916, 38, .
16. Helfferich, F.: Ion-Exchange, Dover Publications Inc., New York 1995.
Justyna JACHUŁA ? M.Sc., graduated from the Faculty of Chemistry, Maria Curie-Skłodowska University in Lublin in 2008. Since 2010 she has been a student of the doctoral course in the Department of Inorganic Chemistry, Maria Curie-Skłodowska University. Her scientific interests: sorption, chelating agents. She is the author of 2 chapters of monographs, 5 papers and several posters presented at national and international conferences.
e-mail: ; phone: +
Zbigniew HUBICKI ? (Sc.D.) Professor, graduated from the Faculty of Chemistry, Maria Curie-Skłodowska University in Lublin in 1969. Scientific interests: hydrometallurgy, chemistry and technology of save elements, methods of inorganic elements separation and environment protection. He is the author of a few chapters in monographs, over 200 scientific papers as well numerous presentations and posters at national and international conferences.
e-mail: ; phone:
